Dr. Mark Shiflett Awarded 50th Patent, Marking Major Milestone in Sustainable Engineering Innovation

Lawrence, Kansas — December 23, 2025 — Dr. Mark B. Shiflett, Foundation Distinguished Professor at the University of Kansas and Director of both the Wonderful Institute for Sustainable Engineering (WISE) and the NSF Engineering Research Center for Environmentally Applied Refrigerant Technology (EARTH), has reached a major career milestone: the issuance of his 50th U.S. patent. This achievement reflects decades of sustained innovation across industry and academia and underscores his contributions to advancing chemical engineering technologies with real-world impact.
Dr. Shiflett’s body of patented work spans a broad range of technologies, from hydrofluorocarbon refrigerant mixtures developed early in his career to enable reduced environmental impact, to novel processes for separations and materials in sustainable energy and chemical processes. Several of his earliest patents were granted during his 28-year tenure at DuPont’s Central Research and Development organization, where he served as a Technical Fellow and helped drive the company’s innovation in fluorochemicals and industrial processes.
At the University of Kansas, Dr. Shiflett has continued this legacy in both teaching and research, mentoring students and leading projects that translate fundamental science into practical technologies. His inventions increasingly focus on ionic-liquid-based separations, advanced purification methods, and sustainable chemical processing techniques that align with global needs for energy efficiency and reduced greenhouse gas emissions.
As the Director of WISE and NSF EARTH, he collaborates with academic, industry, and government partners to advance sustainable refrigerant technologies, train the next generation of engineers, and translate research innovations into solutions that address global environmental and energy challenges.
Dr. Shiflett’s 50th patent represents not only a personal milestone, but also a testament to the long-term value of research-driven innovation and industry engagement.
The full list of Dr. Shiflett’s patents is available on the Shiflett Research Group website.
Clarice M. Sabolay and Dr. Mark Shiflett Publish New Study on Low-GWP Refrigerants to Advance Sustainable Cooling



Lawrence, Kansas — December 8, 2025 — Researchers from the U.S. National Science Foundation Engineering Research Center, Environmentally Applied Refrigerant Technology Hub (EARTH) at the University of Kansas have published a significant new study on next-generation low-GWP refrigerants. The team includes EARTH Graduate Student and Self Fellow, Clarice M. Sabolay; Dr. Mark B. Shiflett, Director of EARTH and Director of the Wonderful Institute for Sustainable Engineering; and Wonderful Institute for Sustainable Engineering graduate, Lokesh S. Valluru.
Their paper, “Model Comparison of Performance, Operating and Capital Cost, and Environmental Impact for HFC-32/HFO-1234yf Mixtures as a Low Global Warming Alternative to R-410A,” was published in Industrial & Engineering Chemistry Research. The study offers one of the most comprehensive evaluations to date of the performance, environmental impact, and cost considerations involved in transitioning from R-410A to more sustainable refrigerants.
With heating, ventilation, air-conditioning, and refrigeration (HVACR) systems accounting for more than 50% of building energy consumption in the United States, improving the environmental impact of refrigerants is a key priority. Traditional refrigerants such as R-410A have high global warming potentials, and global policies, including the U.S. AIM Act and the Kigali Amendment, require a phasedown of these compounds.
EARTH’s new publication closely examines mixtures of HFC-32 and HFO-1234yf, including the widely discussed replacement refrigerant R-454B, alongside pure HFC-32. Using detailed vapor-compression cycle modeling and cost analyses, the authors evaluate how different blend compositions affect energy efficiency (COP), capital and operating costs, heat-transfer behavior, and total equivalent warming impact (TEWI).
Higher HFC-32 compositions, including R-454B and pure HFC-32, deliver higher energy efficiency and lower energy costs compared with R-410A.
These blends also reduce environmental impact, lowering CO₂ emissions through both direct effects (leakage) and indirect effects (energy consumption).
R-454B demonstrates strong heat-transfer performance, while pure HFC-32 provides the highest overall thermodynamic efficiency.
Global adoption of refrigerants such as R-454B and HFC-32 could lead to billions of metric tons of CO₂ emissions avoided each year.
This research provides crucial guidance for regulators, equipment manufacturers, and building system designers as the refrigeration and HVAC industries prepare for broad adoption of A2L refrigerants starting in 2026.
Learn more:
Wonderful Institute for Sustainable Engineering (WISE)
NSF Engineering Research Center for Environmentally Applied Refrigerant Technology (EARTH)
Sarah Walsh Wins KU Excellence in Community, Education, and Leadership (ExCEL) Award

Lawrence, Kansas — November 4, 2025 — The NSF Engineering Research Center for Environmentally Applied Refrigerant Technology (EARTH) proudly recognizes Sarah Walsh, a Chemical Engineering student and member of the Shiflett Research Group, for being named the recipient of the 2025 Excellence in Community, Education, and Leadership (ExCEL) Homecoming Award at the University of Kansas.
Sarah, who is affiliated with both the Wonderful Institute for Sustainable Engineering (WISE) and NSF ERC EARTH, was honored during the KU Homecoming football game on Saturday, where she learned of her selection. The ExCEL Award recognizes two outstanding KU students each year for exceptional leadership, involvement, and commitment to the campus and community.
A B.S. Chemical Engineering major with minors in Business and Biomedical Engineering, Sarah is also a SELF Fellow and an active researcher advancing sustainable refrigerant and cooling technologies through her work with WISE and EARTH.
The WISE community congratulates Sarah on this remarkable achievement and celebrates her leadership, service, and dedication to engineering innovation.
Congratulations, Sarah, on representing WISE, NSF ERC EARTH, and the University of Kansas with excellence!
Learn more:
Wonderful Institute for Sustainable Engineering (WISE)
NSF Engineering Research Center for Environmentally Applied Refrigerant Technology (EARTH)
KU Chemical Engineering PhD Candidate Featured on Journal Cover for Innovative Refrigerant Research

Lawrence, Kansas — October 30, 2025 — Kevin Turner, a PhD candidate in Chemical Engineering at the University of Kansas, was recently recognized by the Journal of Industrial & Engineering Chemistry Research (I&EC Research) for his outstanding scientific and collaborative work. His article, “Methodology Development for the Measurement of Refrigerant Flammability Limits,” was selected for the journal’s cover and highlights a successful research partnership between the University of Kansas and Hudson Technologies to advance the safe and sustainable use of next-generation refrigerants.
The research, co-authored by Turner, Arthur Benson, Michael Lundin, Ed Atchison, and Dr. Mark B. Shiflett from KU, along with collaborators Dave Watson, Steve Campbell, Brian Finney, Glen Khlebutin, and Riyaz Papar from Hudson Technologies, presents a new approach to precisely determine the flammability limits of refrigerants—critical data for ensuring the safe use of low-global-warming-potential refrigerants in next-generation cooling technologies.
The cover art, designed by artist Gil J. Ortiz, highlights Turner’s commitment to developing new refrigerant methodologies grounded in safety and precision.
Turner conducts his research in the Shiflett Research Group within the Wonderful Institute for Sustainable Engineering (WISE) at KU, led by Dr. Mark Shiflett. He is also part of the NSF Engineering Research Center for Environmentally Applied Refrigerant Technology (EARTH), a National Science Foundation funded Engineering Research Center, also directed by Dr. Mark Shiflett, where he works to develop safer and more sustainable refrigerant technologies.
The article appears in Ind. Eng. Chem. Res. (2025) with DOI: 10.1021/acs.iecr.5c01453
Learn more about WISE
Learn more about NSF ERC EARTH
Learn more about Hudson Technologies
Dr. Mark Shiflett and Shiflett Research Group Alumna Dr. Abby Harders Achieve First Patented Membrane-Based Separation of Azeotropic Refrigerant Blend

Lawrence, Kansas — September 22, 2025 — The U.S. Patent and Trademark Office has officially granted U.S. Patent No. 12,427,473, co-developed by Chromis Technologies and KU researchers, led by Dr. Mark Shiflett and graduate alumna Dr. Abby Harders.
This newly issued patent covers a novel class of fluoropolymer-coated hollow fiber membranes capable of separating azeotropic refrigerant blends, a challenge long considered unsolvable. Specifically, the technology enables the selective removal of difluoromethane (HFC-32) from mixtures such as R-410A, paving the way for the first scalable pathway to recycle these high-value compounds.
The global HVAC and refrigeration sector faces a collision of forces: exploding demand for cooling, tightening regulation on high-GWP refrigerants, and the absence of viable recycling solutions for azeotropic blends. Every second, 10 new air conditioners are sold worldwide, adding to the massive consumption of refrigerants. Yet blends like R-410A cannot be separated by conventional methods such as distillation, forcing most end-of-life refrigerants to be vented or destroyed. This results in valuable compounds being lost and significant emissions released into the atmosphere.
The patented technology addresses this long-standing challenge. The membrane system uses a submicron layer of custom fluoropolymer coated onto hollow fibers to selectively permeate HFC-32 while rejecting HFC-125. This innovation delivers single-pass purities above 95 mol% HFC-32 and is produced through a reel-to-reel coating process that enables scalable manufacturing of defect-free fibers. By offering a non-thermal, energy-efficient solution for reclaiming refrigerants, it opens the door to real-world recycling and reuse.
“This is the first time an azeotropic refrigerant blend has been effectively separated into its pure components using a scalable membrane solution,” said Dr. Mark Shiflett, Director of the Wonderful Institute for Sustainable Engineering at KU.
Building on this foundation, the work has been spun out into Icorium Engineering Company, a startup dedicated to commercializing membrane and distillation-based refrigerant separation technologies. Icorium is already developing industrial-scale modules to serve reclaimers, HVAC manufacturers, and sustainability-minded partners worldwide.
“This patent represents not only scientific innovation but also a practical route to circularity in refrigerants,” said Dr. Abby Harders, whose doctoral research at KU contributed to the breakthrough.
With regulatory deadlines approaching, including EPA requirements for recycled refrigerants by 2028, this solution arrives at the perfect moment. It demonstrates how innovations in polymer science can unlock scalable climate solutions, turning end-of-life refrigerants into reusable resources and dramatically reducing emissions across the cooling sector.
About the Wonderful Institute for Sustainable Engineering (WISE)
The Wonderful Institute for Sustainable Engineering at the University of Kansas is committed to advancing breakthrough technologies that address the world’s most pressing environmental challenges. Through interdisciplinary research, education, and industry partnerships, WISE fosters innovation that accelerates the transition to a more sustainable future.
About Icorium Engineering Company
Icorium Engineering Company is a clean-technology startup focused on commercializing advanced membrane and distillation systems to enable true circularity in refrigerants. By transforming patent-protected innovations into industrial-scale solutions, Icorium empowers HVAC systems and reclaimers to recover high-value refrigerants, reduce emissions, and support sustainable cooling at scale.
Dr. Mark Shiflett Reaches 11,000 Career Citations

Lawrence, KS — September 1, 2025 — Dr. Mark B. Shiflett, Foundation Distinguished Professor of Chemical & Petroleum Engineering at the University of Kansas, has reached a remarkable academic milestone of 11,000 career citations with H-Index: 51 and i10-Index: 145 on September 1, 2025.
Dr. Shiflett’s research spans sustainable refrigerants, gas separations, and advanced materials, driving innovation at the intersection of science, engineering, and industry. His contributions have shaped global conversations on sustainable cooling & heating technologies, while also mentoring the next generation of engineers through his leadership at KU and as Director of both the Environmentally Applied Refrigerant Technology Hub (EARTH) NSF Engineering Research Center and the Wonderful Institute for Sustainable Engineering (WISE).
Dr. Shiflett’s scholarship continues to make lasting impact across academia, industry, and society. This milestone reflects not only the reach of his research but also the collaborative spirit of his students, colleagues, and partners.
Congratulations to Dr. Shiflett on this outstanding achievement!
Dr. Mark Shiflett Presents Plenary Lecture at International Fluorine Chemistry Symposium

Lisbon, Portugal — August 4, 2025 — Dr. Mark Shiflett was honored to serve as an invited Plenary Speaker at the 21st European Symposium on Fluorine Chemistry (ESFC-21) in Lisbon, Portugal. The symposium, a premier international gathering of scientists advancing the frontiers of fluorine chemistry, provided an ideal platform to highlight the vision and research of EARTH, a newly funded Gen-4 National Science Foundation Engineering Research Center called “Environmentally Applied Refrigerant Technology Hub (EARTH)”. Lead by the University of Kansas and in partnership with University of Notre Dame, University of Maryland, University of Hawaiʻi, Manoa, Lehigh University and the University of South Dakota, EARTH focuses on developing a circular refrigerant economy.
Mark Shiflett, who serves as the Center Director, introduced the global scientific community to the mission and goals of the EARTH ERC in his address entitled “Environmentally Applied Refrigerant Technology Hub (EARTH)”. The talk highlighted the Center’s multidisciplinary approach to advancing next-generation refrigerant technologies, addressing urgent atmospheric challenges, and training the future workforce in sustainable cooling solutions.
The European Symposium on Fluorine Chemistry is renowned for bringing together researchers from academia, industry, and government to exchange breakthroughs across inorganic, organic, materials, and applied fluorine chemistry. EARTH’s presence at this year’s symposium underscores the Center’s growing international engagement and leadership in refrigerant and cooling innovation.
By sharing EARTH’s work on an international stage, the Center continues to expand its network, inspire new partnerships, and position itself at the forefront of next generation cooling technologies.
Abbie Peters Named Awardee at the 2025 Future Leaders in Chemical Engineering Symposium

Lawrence, KS — August 29, 2025 — Undergraduate Researcher, Abbie Peters, working with Dr. Mark Shiflett, Distinguished Foundation Professor at the University of Kansas – Chemical & Petroleum Engineering Department, has been selected as an awardee for the 2025 Future Leaders in Chemical Engineering Symposium, a premier national event hosted by the Department of Chemical and Biomolecular Engineering at North Carolina State University.
Abbie is the third Undergraduate Researcher in Dr. Mark Shiflett’s Research Group to be selected for this prestigious award. Previous Shiflett Research awardees include Hannah Uhl and Greta Olsen.
The symposium recognizes the most promising early-career researchers in the field of chemical engineering and provides an elite forum for honorees to present their work, exchange ideas with peers, and engage with academic and industry leaders.
Peters was chosen following a highly competitive application process that drew an exceptional pool of candidates from across the United States. Her selection underscores both the quality and impact of her research and her potential to contribute to the advancement of chemical engineering.
The Future Leaders in Chemical Engineering Symposium will be held at NC State University October 9-10, 2025, offering awardees opportunities to connect with distinguished faculty, established professionals, and other emerging leaders in the field.
As part of the symposium, Peters will deliver a presentation on her research and participate in networking sessions and professional development activities designed to highlight the next generation of leaders in the discipline.
For more information on the symposium, please visit: Future Leaders in Chemical Engineering
For more information on the Wonderful Institute for Sustainable Engineering, please visit: Wonderful Institute for Sustainable Engineering
Dr. Mark Shiflett among 6 KU innovators selected for Rock Chalk Ready commercialization program

Published: 08/19/2025
The University of Kansas Center for Technology Commercialization (KUCTC) has selected six promising research projects for its inaugural Rock Chalk Ready program, a universitywide initiative designed to mature early-stage innovations and position them for commercialization success.
Supported by a FORGE grant from the Kansas Department of Commerce, Rock Chalk Ready reflects a collaborative One KU approach, inviting participation from innovators on KU’s Lawrence and Medical Center campuses. The program aims to de-risk technologies and business concepts by providing funding, guidance and connections to resources across KU’s innovation ecosystem.
“We were thrilled to see the enthusiastic response from our research community to the Rock Chalk Ready program,” said Cliff Michaels, director of KUCTC. “We expect these funds will help these innovators make meaningful advances over the next six to 12 months and remove some of the risks inherent in early-stage innovations.”
The program received 25 proposals from across the university, demonstrating strong interest and need for early-stage innovation support. A panel of internal and external experts reviewed proposals, with six ultimately selected for funding. Projects range from novel therapeutics and medical devices to industrial and agricultural innovations, highlighting the breadth of cutting-edge research happening at KU.
Funded Rock Chalk Ready projects:
- Alan Allgeier – Technologies to Enhance Corn Oil Extraction During Ethanol Production. Allgeier is a professor of chemical & petroleum engineering.
- Michael Hageman – Prodrug Formulations for Oral Testosterone Replacement Therapy. Hageman is the Valentino J. Stella Distinguished Professor of Pharmaceutical Chemistry.
- Divya Kamath – A Novel Therapeutic for Multiple Sclerosis. Kamath is a research assistant professor of cancer biology.
- Simon Patton – A Medical Device for Improved Hysterectomy Surgeries. Patton is a clinical assistant professor of obstetrics & gynecology.
- Shyam Sathyamoorthi – Antifungal and Antibacterial Agents for Industrial Agricultural Use. Sathyamoorthi is an associate professor of medicinal chemistry.
- Mark Shiflett – Novel Acids for Use in the Fuel and Detergent Industries. Shiflett is a Foundation Distinguished Professor of Chemical & Petroleum Engineering.
The Rock Chalk Ready program embodies a cross-campus partnership where KU Innovation Park, the KU Office of Economic Development and KUCTC all collaborated in securing the FORGE grant. KUMC’s research administration team is helping manage the initiative. The KU School of Business is also contributing by pairing business students with the selected teams to assist with market analysis and commercialization planning.
Rock Chalk Ready projects will continue through summer 2026.
Read full article: https://wise.ku.edu/news/article/6-ku-innovations-selected-for-rock-chalk-ready-commercialization-program
Wonderful Institute for Sustainable Engineering Research Team Awarded $31,000 for Sustainable Catalyst Innovation

Published: July 21, 2025
The KU Center for Technology Commercialization (KUCTC) has announced that a Wonderful Institute for Sustainable Engineering research team has been selected for funding through the Rock Chalk Ready Opportunity Development Program. The awarded project is led by Dr. Tommy Poskin, a postdoctoral researcher at the Wonderful Institute for Sustainable Engineering, along with Professors Aaron Scurto and Mark Shiflett. The team will receive $31,000 to advance the development of safer, more sustainable acid catalysts for industrial use.
The Rock Chalk Ready funds will be used to support lab-scale scale-up, performance testing, and potentially early business development activities.
For more information about the Rock Chalk Ready program or to explore commercialization resources, contact KUCTC@ku.edu.
Rock Chalk!
Kevin Turner Successfully Passes Preliminary Exam with Insightful Presentation on Refrigerant Flammability

Published: July 7, 2025
Kevin Turner, a Graduate Student working in the Shiflett Research Group, has successfully passed his preliminary examination, marking a major milestone in his academic journey. His presentation, titled “Refrigerant Flammability Metrics, Methodologies, and Modeling,” showcased a deep understanding of critical safety and performance factors in refrigerant technologies.
In his talk, Turner addressed the growing importance of flammability analysis as industries transition toward low-GWP (global warming potential) refrigerants – many of which pose new safety challenges. His research focused on quantifying flammability using well-defined metrics such as lower flammability limits (LFL), upper flammability limits (UFL), and minimum ignition energy (MIE).
Turner also detailed a variety of testing methodologies used to assess these properties. These included controlled ignition experiments and simulation-driven analysis to complement laboratory results. His work highlighted the complexities in creating standardized flammability profiles and the need for unified testing protocols.
Faculty members and committee participants commended Turner for both the depth of his technical content and the clarity of his delivery. His ability to bridge experimental data with computational modeling was noted as a key strength of his work.
With the preliminary exam now behind him, Turner will continue developing his research toward a full dissertation. His future work is expected to contribute significantly to the field of refrigerant safety, particularly as the industry navigates stricter environmental regulations and evolving safety standards.
Turner’s successful exam represents not only a personal achievement but also a promising step forward for innovation in sustainable cooling technologies.
Congratulations, Kevin!
Julia Espinoza Mejia Successfully Passes her PhD Comprehensive Exam
Published: June 13th, 2025

Julia Espinoza Mejia of the Shiflett Research Group has successfully passed her PhD Comprehensive Exam! Julia’s proposal was titled “Ionic Liquid-Based Extractive Distillation for Sustainable Refrigerant Recovery”. Congratulations to Julia for her success in passing her Comprehensive Exam!!🎉🎉🎉
Abdulrhman Arishi Successfully Passes his PhD Comprehensive Exam
Published: May 16th, 2025


Abdulrhman Arishi of the Shiflett Research Group has successfully passed his PhD Comprehensive Exam! Arishi’s proposal was titled “Separation of Hydrofluorocarbon Mixtures using Extractive Distillation with an Ionic Liquid Entrainer”. Congratulations to Arishi for his success in passing his Comprehensive Exam!! 🎉🎉🎉
Dr. Abby Harders and Dr. Andrew Yancey-Jardon win prestigious awards at the Chemical and Petroleum Engineering Banquet on May 10, 2025
Published: May 12th, 2025

Dr. Abby Harders who received her PhD in Chemical Engineering in July 2024 received the Frank Bowdish Outstanding PhD Award in Chemical Engineering and Dr. Andrew Yancey-Jardon who received his PhD in Chemical Engineering in December 2024 received the Maloney Award for Writing in Chemical Engineering.
Arthur Benson was awarded the 2025 Outstanding Presentation Award at the 28th Annual Undergraduate Research Symposium
Published: May 9th, 2025

LAWRENCE — More than 130 University of Kansas undergraduate students participated in the 28th annual Undergraduate Research Symposium, which featured oral and poster presentations as well as artists’ talks and creative displays from many disciplines.
This year’s symposium took place during Undergraduate Research Week, which was April 21-25.
Of those presenters, 34 students received Outstanding Presentation Awards, while three group presentations were also honored. Award recipients each receive a $50 award per presentation.
The 2025 Outstanding Presentation Award winners are listed by name, major, hometown presentation name, mentor and mentor department:
Individuals
Arthur Benson, chemical engineering, Lawrence; “Enhancing the Accuracy and Reproducibility of Lower Flammability Limit Testing for Refrigerants,” mentored by Mark Shiflett and Kevin Turner, chemical & petroleum engineering.
Read the full article here: https://news.ku.edu/news/article/ku-celebrates-undergraduate-research-announces-presentation-winners
Emmanuel Ababio Receives the Prestigious Chancellor’s Graduate Fellowship Award
Published: April 30th, 2025

Congratulations to Emmanuel Ababio, PhD student in the Wonderful Institute for Sustainable Engineering and the Department of Chemical & Petroleum Engineering for receiving the prestigious Chancellor’s graduate fellowship award. This award recognizes outstanding graduate students who have demonstrated exceptional academic achievement, research potential, and leadership in their fields of study. Awarded through a highly competitive selection process, these fellowships not only celebrate excellence, but also provide crucial financial support that enables recipients to focus fully on their research and academic goals. Emmanuel is advised by Foundation Distinguished Professor Mark Shiflett and is working with Dr. Andrew Yancey-Jardon and Dr. David R. Corbin to develop porous materials for separation of azeotropic refrigerant mixtures.
CJ Ponge is Selected to Present his Research at the Capitol Graduate Research Summit
Published: April 30th, 2025

Congratulations to Charles “CJ” Ponge, PhD student in the Wonderful Institute for Sustainable Engineering and the Department of Chemical & Petroleum Engineering for being selected to present his research at the Capitol Graduate Research Summit (CGRS). The CGRS is an annual event where selected graduate students from all seven Kansas Board of Regents (KBOR) universities present their impactful research to state legislators and officials in the State Capitol Building in Topeka, Kansas. This summit showcases the significant contributions of our graduate student community. CJ is advised by Foundation Distinguished Professor Mark Shiflett and is working with Dr. David R. Corbin to develop porous materials for removal of per- and poly-fluoroalkylsubstances (PFAS) from contaminated water and soil.
KU announces new 2025-2029 Self Graduate Fellows
Published: April 28, 2025

LAWRENCE – Twenty doctoral students have been selected to receive the University of Kansas’ prestigious Madison and Lila Self Graduate Fellowship for the 2025-2026 academic year. This incoming group of fellows is the largest cohort in the history of the Self Graduate Fellowship, bringing the total number of beneficiaries to over 220 students throughout the program’s history. In fall 2025, the fellowship reaches 57 total current fellows, making it the largest fellowship size ever.
The Self Graduate Fellowship’s mission is to identify and recruit exceptional doctoral students who demonstrate the promise to make significant contributions to their fields and society as a whole. The total value of the four-year doctoral fellowship exceeds $225,000.
The fellowship is a four-year package awarded to incoming and first-year doctoral students who demonstrate leadership, initiative and passion for achievement. The fellowship covers full tuition and fees, provides graduate research assistant support of $38,000 per year, a $12,000 professional development award, $5,000 start-up award, $3,000 textbook and technology award, and a robust professional development program.
The Fellow Development Program provides general education and training in communication, management, innovation, policy and leadership to assist Self Graduate Fellows in preparation for future leadership roles. The development program complements the specialized education and training provided in doctoral programs.
The late Madison and Lila Self launched and permanently endowed the Self Graduate Fellowship in 1989, motivated by their strong belief in the vital importance of developing leadership for tomorrow. Madison Self was a 1943 KU graduate in chemical engineering. Lila Self attended KU with the Class of 1943.
The new Self Graduate Fellows for the 2025-2029 cohort:
Claire Sabolay, of Belleville, Illinois: bachelor’s degree in chemical & petroleum engineering, KU; first-year doctoral student in chemical & petroleum engineering.
Read the full article here: https://news.ku.edu/news/article/ku-announces-new-2025-2029-self-graduate-fellows
Published: Issue 1, 2025
Way Cool: KU Leads Plant-Protecting Research on Refrigerants
A new federally funded center, directed by School of Engineering professor Mark Shiflett, seeks to boost the Kansas economy while driving a heating and cooling revolution.

As kickoff celebrations go, the launch of a research center dedicated to refrigerant technology might seem anything but cool. Refrigerants, the chemical compounds that keep our homes, workplaces, automobiles, medicines and food (even that ancient beer fridge in the garage) at ideal temperatures are important, no doubt. But Marching-Jayhawks-in-full-game-day-regalia important?
Believe it.
As the daylong event marking the opening of KU’s newest research center gets underway Nov. 7 at the Burge Union, a succession of high-profile speakers step to the microphone to laud the potential of the Environmentally Applied Refrigerant Technology Hub, a massive, six-university venture led by the KU School of Engineering. Funded by a $26 million federal grant, EARTH will tackle a major challenge facing the planet: how to curb one of the largest contributors to global warming—namely, the greenhouse gases and energy demand produced by our climate control systems—at a time when rising temperatures are fueling ever greater need for those systems worldwide.
Leading off the speakers, Chancellor Doug Girod hails EARTH as “game-changing in so many ways,” calling it nothing less than an ambitious attempt to develop a new industry that could boost the Kansas economy.
U.S. Sen. Jerry Moran, c’76, l’82, casts the center as a remedy to the brain drain that siphons many of Kansas’ best and brightest, who often leave the state to seek jobs. “Today is one more opportunity for us to expand the opportunities for students and business and industry and increase the capabilities of our country to deal with environmental issues,” Moran says. “So it’s a win-win-win in my world, and I’m delighted to be a part of it.”
Sethuraman Panchanathan, director of the National Science Foundation, which awarded the five-year grant that is among the largest in the University’s history, touts the effort’s “huge impact” on humanity. “This project is phenomenal,” Panchanathan enthuses. “What it is going to do is to unleash scientific ideas, to unleash innovation, to unleash the technology of the future and—most importantly—the industries of the future and therefore the jobs of the future, having an impact on economic, societal as well as national security—all at the same time.”
EARTH is one of only 19 Engineering Research Centers currently funded by the NSF in the fields of advanced manufacturing, energy and the environment, health, and microelectronics. The result of a highly competitive, four-year process that required a 1,000-page application, a site visit by NSF teams, and extensive collaboration among KU and its five research university partners across the country (plus multiple community and vocational colleges across the state), EARTH is headed by KU Foundation Distinguished Professor Mark Shiflett, a former DuPont researcher who joined the department of chemical and petroleum engineering in 2016 as the University’s 12th and final Foundation Distinguished Professor.
Taking his turn at the microphone, Shiflett notes the multiyear whirl of activity that has led to this celebratory moment, including consultations with faculty and students from the schools joining KU on the project, as well as with stakeholders in the heating, ventilation, air conditioning and refrigeration (HVACR) industry, dozens of whom are attending today. As the person ultimately responsible for delivering on the colossal promise of EARTH, Shiflett delivers his remarks in a more sober and measured tone than the speakers who preceded him. But his confidence—as he makes clear exactly what the stakes are—is just as high.
“We are going to help solve some of the most challenging engineering problems with transitioning this industry to more environmentally friendly and energy-efficient refrigerants,” Shiflett says, speaking slowly and softly. “We named it EARTH. We, all of us—all of us—live on a planet that’s called Earth, and we have to take care of it. It’s our home.”
Read the full article here: https://kansasalumnimagazine.org/magazine-article/ku-engineering-refrigerant-technology/
KU Innovation Park welcomes Dr. Mark Shiflett to its Board of Directors

KU Innovation Park is excited to welcome three new members to its board of directors. Their combined expertise will enhance the Park’s mission to promote innovation, support high-growth companies, and drive regional economic impact. As KU Innovation Park expands, these new board members offer valuable insights that will help shape its future and its developing entrepreneurial ecosystem.
The three new members are:

Michele Hammann, CPA/PFS, CVA – Chief Strategy Officer, SSC CPAs + Advisors
Michele received her Master of Accounting and Information Systems from the University of Kansas in 2001. She is a member of the American Institute of CPAs, the Kansas Society of CPAs and the National Association of Certified Valuation Analysts. She is the past chair of the board for The Chamber of Lawrence and a past president of Junior Achievement of Douglas County. In 2019, Michele received the KSCPA/AICPA Women to Watch Experienced Leader Award. Michele will serve as the board’s treasurer.

Mark Shiflett, Ph.D., P.E. – Co-founder & CSO, Icorium Engineering & Distinguished Foundation Professor, KU School of Engineering
Dr. Mark Shiflett is a Distinguished Foundation Professor in the School of Engineering at the University of Kansas (KU), where his research focuses on developing environmentally friendly, energy-efficient processes and products for the chemical industry. He retired from the DuPont Company after 28 years in 2016 as a Technical Fellow in the Central Research and Development Organization at DuPont’s Experimental Station in Wilmington, Del. Mark is an inventor on 46 U.S. patents and has published over 130 articles on his research at DuPont. He is also the co-founder and chief science officer of Icorium Engineering Company, a KU spin-out company located at KU Innovation Park that is revolutionizing refrigerant reclamation with efficient, complete separation of even the most complex mixes.

Steven Stites, M.D., F.A.C.P., F.A.A.C.P – Executive Vice President, Clinical Affairs & Chief Medical Officer, KU Medical Center
Dr. Steve Stites joined the University of Kansas Hospital’s leadership team as senior vice president of clinical affairs in February 2012 and became executive vice president and chief medical officer for the health system in July 2018. He serves a dual role as vice chancellor for clinical affairs at the University of Kansas Medical Center. Dr. Stites received his medical degree from the University of Missouri-Columbia. He completed his residency and chief residency in internal medicine at the University of Rochester and a fellowship at the University of Kansas Medical Center.
The new board members are Class B board members, appointed by the Park’s Class A members. Class A members represent each of the Park’s four founding partners – the city of Lawrence, Douglas County, The Chamber of Lawrence and the University of Kansas. The new members will serve a three-year term, which began at the start of the calendar year.
“One of the strengths of KU Innovation Park is the expertise, vision, and leadership of those who guide our mission,” said Adam Courtney, CEO of the Park. “Michele, Mark, and Steve offer invaluable perspectives that will enhance our ability to support bioscience and technology companies, promote research commercialization, and drive long-term economic growth.”
The Park is immensely grateful for the board service of Dr. Val Stella, Bob Johnson, and Dr. Robert Simari. Stella and Johnson have been involved with the Park since its inception in their respective roles at KU and the Douglas County Commission. Their board terms concluded at the end of 2024.
Read full article: https://kuinnovationpark.com/ku-innovation-park-welcomes-three-new-members-to-its-board/
Published on: 02/20/2025
Research yields eco-friendly way to separate, recycle refrigerants tied to climate crisis

LAWRENCE — A scholarly report in the journal Science Advances from researchers at the University of Kansas shows a new eco-friendly method for separating the chemicals found in common refrigerants for easier recycling at industrial scale.
“The motivation of this work is to enable separation of highly complex gaseous refrigerant mixtures,” said lead author Abby Harders, who performed the research as a KU doctoral student in the research group of co-author Mark Shiflett, Foundation Distinguished Professor of Chemical and Petroleum Engineering. “This effort has been driven by climate legislation phasing out certain hydrofluorocarbon (HFC) refrigerants.”
The paper’s key innovation uses membranes — amorphous fluorinated polymers, to be specific — that efficiently isolate complex refrigerant mixtures. Other separation methods, like distillation, are less effective because of the complex composition of the mixtures. Harders said the membranes are fabricated to allow some gases to pass through while restricting others — resulting in effective purification.
To demonstrate the technology could scale to industrial viability, the team — including many associated with KU’s Wonderful Institute for Sustainable Engineering — developed a custom-coating process to create submicron coatings on the membrane’s porous supports, creating composite hollow fibers. The results show a functional prototype, proving the technology’s usefulness to firms engaged in refrigerant recovery and reuse.
Read full article: https://news.ku.edu/news/article/research-yields-eco-friendly-way-to-separate-recycle-refrigerants-tied-to-climate-crisis
Published: 02/12/2025
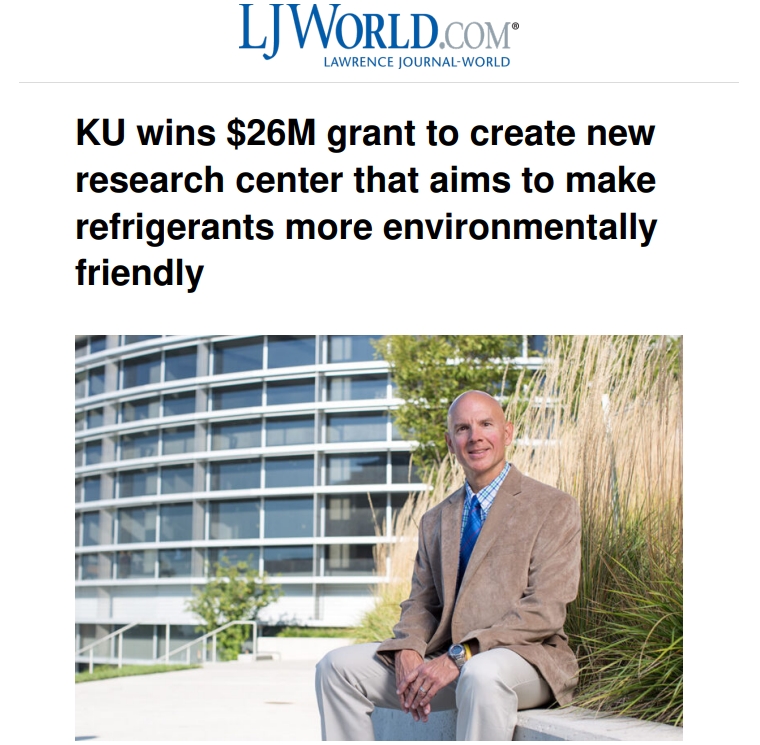
Mark Shiflett, Foundation Distinguished Professor in the Department of Chemical & Petroleum
Engineering and director of the Wonderful Institute for Sustainable Engineering, University of Kansas,
will lead the EARTH Center.
The University of Kansas has won a $26 million federal grant — one of its
largest in history — to help develop new technologies that will make everything
from air conditioners to refrigerators more environmentally friendly.
The National Science Foundation has awarded KU $26 million to develop an
Environmentally Applied Refrigerant Technology Hub. The cutting edge
research center will work to develop new types of refrigerants that are kinder to
the environment.
“It will be a big research center here,” said Mark Shiflett, who will serve as the
center’s director and is currently a KU distinguished professor in chemical and
petroleum engineering.
Work across the world is already underway to develop and implement a new
class of refrigerants that don’t contribute to global warming in the same way
that the current class of products do. Refrigerants — the fluids used in all types
of cooling devices — are estimated to account for about 8% of global
greenhouse gas emissions currently.
Development of those new products is expected to make the refrigerant
business a $1 trillion industry in the future, Shiflett said in an interview with the
Journal-World. KU is now positioned well to be one of the U.S. research
leaders in that huge field.
Shiflett said 28 private companies in the heating and cooling industry have
already said they want to join the center because they think KU and its
partners will be key in making the industry more sustainable.
“And these are big HVAC companies,” Shiflett said of the industry partners,
which have not yet been publicly named.
The center, which KU has given the acronym of EARTH, will be located in
existing space inside the engineering school complex on KU’s main campus,
Shiflett said. The center, which will be operational this fall, will have at least 12
faculty and research staff members on the KU campus, plus the university will
hire several administrative positions to help oversee the entire program, which
includes work that will be done at six partner research institutions across the
country.
While KU is the lead institution on the project, it has partners at the University
of Notre Dame, University of Maryland, University of Hawaii, University of
South Dakota and Lehigh University. Shiflett said the entire project is expected
to include 42 faculty members from 16 academic disciplines. It also will partner
with 22 community and technical colleges to help train future employees for the
refrigeration industry. The KU campus will serve as the overall headquarters for
the enterprise, Shiflett said.
At the helm of the operation will be Shiflett, who already is a pioneer in the
industry. Shiflett spent nearly 30 years at the chemical giant DuPont, where he
was the inventor of a new refrigerant that became used around the world in
supermarket freezers. That product had more than $1 billion in sales for
DuPont at the time Shiflett left the company in 2016 to join the faculty at KU.
Shiflett is currently the director of the Wonderful Institute for Sustainable
Engineering, another KU center that recently has been in the news. As the
Journal-World reported in November, KU named the center after The
Wonderful Company, which is the $5 billion privately held California company
that is the world’s largest grower of pistachios, owner of the Halo brand of
mandarin oranges, and the owner of the Fiji water brand, among others.
Importantly, the owners of the Wonderful Company — Stewart and Lynda
Resnick — are some of the largest university donors in the country, with a
particular emphasis on funding sustainability projects. The couple in 2019
made a $750 million pledge to the California Institute of Technology, which
houses a sustainability institute named after the family.
With KU having its own sustainable engineering institute already in place, the
university is now getting another level of support by winning the NSF grant,
which more than a hundred other universities sought for a host of advanced
research projects.
KU and state leaders on Wednesday were touting the importance of the federal
grant award.
“Working closely with industry partners, EARTH will have the resources and
expertise to solve the technical, environmental and economic challenges
required to create a sustainable refrigerant lifecycle that will benefit Kansans,
the nation and the world,” KU Chancellor Douglas Girod said in a press
release. “In doing this work, the center is a prime example of how the
University of Kansas is driving economic development in Kansas.”
U.S. Sen. Jerry Moran, R-Kansas, is a member of the Senate Appropriations
subcommittee that funds the NSF. He said the grant is an important one in
keeping the United States competitive in an important industry.
“This new research center will allow Kansans to lead the way in developing the
next generation of refrigerant technology, increasing U.S. competitiveness in an
important technology and industry,” Moran said via a press release.
For Shiflett, the grant is a major milestone years in the making, he told the
Journal-World.
“It is a dream come true,” Shiflett said. “I really love working with students and
helping get their careers started.”
Some of those students will be working to replace the very invention that
Shiflett brought to the industry while at DuPont. While that class of refrigerant
was a major environmental upgrade at the time, new technologies likely can
produce even more environmentally friendly products. Plus, the center will be
looking to break ground on how to recycle refrigerants and reduce energy
usage in the industry, among other projects.
Shiflett is now looking forward to students at the center becoming the highlight
of his career, even over and above the 46 patents he’s been awarded for
inventions.
“I kind of see in the early part of my career I was fortunate to be able to invent
and develop products,” Shiflett said. “And now, in the latter part of my career,
I’m fortunate to be able to develop people.”
Read full article: https://www2.ljworld.com/news/ku/2024/aug/21/ku-wins-26m-grant-to-create-new-research-center-that-aims-to-make-refrigerants-more-environmentally-friendly/
Published on: 08/21/24
WISE participates in the Big Event






October 5, 2024
A group of chemical engineering students and staff from the Wonderful Institute for Sustainable
Engineering at the University of Kansas (WISE-KU) came together again for the KU Big Event. Led by
Chemical Engineering Graduate Student, Claire Sabolay, we met on a warm sunny morning at Lauralyn
Bodie’s property (1534 Wedgewood Drive). She had everything ready for us and we cleared vines and
limbs, mulched, and even cut down a few small trees. Lauralyn teaches Italian at the University of Kansas.
The group enjoyed spending time with her and her dog August. A nice break from doing homework, writing proposals, and preparing for exams. Thank you for your service!
Claire Sabolay – KU PhD graduate student, Chemical Engineering
Yuniva Mendoza-Apodaca – KU PhD graduate student, Chemical Engineering
Emmanuel Ababio – KU PhD graduate student, Chemical Engineering
Sarah Dixon – KU Senior, Chemical Engineering
MJ Jones – KU Freshman, Chemical Engineering
Joey Blanchett – KU Staff, Wonderful Institute for Sustainable Engineering
Mark Shiflett – KU Professor, Chemical Engineering
Published on: 10/05/24
CJ Ponge Successfully Completes Preliminary Exam

CJ Ponge, Shiflett Research Graduate Student, successfully completed his Preliminary Exam, “Zeolites for the Aqueous Sorption of PFAS” today.
Congratulations, CJ!
Published on: 08/19/24
Prof. Mark Shiflett Reaches 10,000 Citations

Congratulations to Prof. Mark B. Shiflett who obtained 10,000 citations on June 29, 2024 for his publications and scholarship according to Google Scholar: Citations: 10,000 with H-Index: 50 and i10-Index: 129
Congratulations, Dr. Shiflett!
Published on: 6/29/24
Abby Harders Successfully Defends Thesis with Honors

Congratulations to Abby Harders, who successfully defended her thesis “Development of Membrane Technology for the Separation of Azeotropic Refrigerants” with honors today.
Congratulations Dr. Harders!
Published on: 7/22/24
Andrew Yancey-Jardon publishes article in Chemical Engineering Journal


Andrew Yancey-Jardon published an article entitled “A new activity coefficient model for describing adsorbed phase nonidealities” in collaboration with David R. Corbin and Mark B. Shiflett in Chemical Engineering Journal. The study introduces a new adsorbed phase activity coefficient model, the SPLINT model, for describing nonideal multicomponent systems.
Congratulations, Andrew!
Published on: 06/27/24
CJ Ponge publishes article in I&EC Research


CJ Ponge, in collaboration with Nathaniel P. Sheehan, David R. Corbin, Edward Peltier, Justin M Hutchison and Mark B. Shiflett, publish manuscript entitled “Zeolites for Sorption of PFAS from Water” in I&EC Research.
Congratulations, CJ!
Published on: 06/26/24
Abby Harders participates in Spring 2024 Graduation and Doctoral Hooding ceremony at the University of Kansas

Abby Harders participated in the Spring 2024 graduation and doctoral hooding ceremony at the University of Kansas. She will be defending her PhD thesis on July 16.
Congratulations Abby!
Published on: 05/20/2024
Dr. Kalin R. Baca receives “Most Outstanding PhD Researcher in the School of Engineering at the University of Kansas”

Dr. Kalin R. Baca received the “Most Outstanding PhD Researcher in the School of Engineering at the University of Kansas” at the Graduation Ceremony on May 11, 2024.
Congratulations, Kalin!
Published on: 05/20/2024
Abdulrhman Arishi publishes article in Industrial & Engineering Chemistry Research

Abdulrhman Arishi published the research article entitled, “Separation of Azeotropic Refrigerant Mixtures: R-450A, R-456A, R-515B, and R-516A Using Phosphonium- and Imidazolium-Based Ionic Liquids” in Industrial & Engineering Chemistry Research (citation: Ind. Eng. Chem. Res. 2024, 63, 6754-6765, DOI: 10.1021/acs.iecr.4c00531).
Congratulations, Arishi!
Published on: 05/07/2024
Hannah Uhl receives Outstanding Academic Achievement and Academic Honors Awards

Hannah Uhl, Senior Undergraduate Researcher, working with PhD graduate student, Abby Harders, received the Outstanding Academic Achievement Award (GPA 3.95 or higher) and the Academic Honors Award (GPA 3.5 or higher) at the KU Chemical and Petroleum Engineering Awards ceremony on May 4, 2024.
Congratulations, Hannah!
Published on: 05/07/2024
Tiana Bish receives Academic Honors Award

Tiana Bish, Senior Undergraduate Researcher, working with PhD graduate student, Andrew Yancey, received the Academic Honors Award (GPA 3.5 or higher) at the KU Chemical and Petroleum Engineering Awards ceremony on May 4, 2024.
Congratulations Tiana!
Published on: 05/07/2024
Hannah Uhl receives Most Outstanding Senior in Chemical Engineering

Hannah Uhl was chosen as the University of Kansas “Most Outstanding Senior in Chemical Engineering” at the KU Chemical and Petroleum Engineering Awards ceremony on May 4, 2024. Hannah will be attending Massachusetts Institute for Technology in the Fall to obtain a PhD in Chemical Engineering.
Congratulations, Hannah!
Published on: 05/07/2024
Dr. Kalin Baca receives Frank Bowdish Outstanding PhD in Chemical Engineering Award

Dr. Kalin R. Baca, a recent graduate from the KU Chemical Engineering program was named the “Frank Bowdish Outstanding PhD in Chemical Engineering” at the KU Chemical and Petroleum Engineering Awards ceremony on May 4, 2024. Kalin is now the Chief Operating Officer at Icorium Engineering Company.
Congratulations, Kalin!
Published on: 05/07/2024
Morela Chapman wins awards at ChemE Undergraduate Research Symposium

Shiflett Research Undergraduate Researcher, Morela Chapman, won 2nd place in the category of up to 2 semesters of research and the people’s choice award in the ChemE Undergraduate Research Symposium organized by KU AIChE last Saturday, April 20th.
Published on: 04/22/2024
Icorium Engineering Company Wins Big at 2024 Rice Business Plan Competition (RBPC) in Houston

Dr. Kalin Baca, co-founder and Chief Operating Officer for Icorium Engineering Company, and Abby Harders, University of Kansas Ph.D. student and part-time Icorium R&D Engineer, competed in the Rice Business Plan Competition (RBPC) held by the Rice Alliance for Technology and Entrepreneurship at Rice University in Houston, April 4-6. Baca completed her Ph.D. in Chemical Engineering at KU in 2023 under the supervision of Foundation Distinguished Professor Mark B. Shiflett. Shiflett, who is Icorium’s Co-Founder and Chief Science Officer, is also Harders’ Ph.D. advisor and accompanied the team to Houston along with Icorium’s Chief Strategy Officer, Erik Blume.
Over three days, Baca and Harders pitched through multiple rounds of intense competition, refining their pitch based on the judges’ feedback as they made it to the 15-team semifinals on Friday, and then on to the 7-team final round on Saturday. Icorium finished fifth-place overall, winning nearly $182,000 in investments and other cash and in-kind prizes. “All of the teams presenting at the competition were really impressive, and even making it to the final round was an incredible experience,” said Baca. “The prize money will make a huge difference for the company at this stage, and we’ve made important connections with several investors who are interested in helping us succeed down the road. We were also thrilled to learn that we were the first team to compete in the RBPC from the University of Kansas.”
The RBPC is one of the country’s premier pitch competitions and showcases the best university startups from around the world. The competition, entering its 24th year, gives collegiate entrepreneurs real-world experience to pitch their startups, enhance their business strategy, and learn what it takes to launch a successful company. This year, more than 450 teams applied and 42 were selected to pitch their technologies for more than $1.5 million in cash and prizes. The RBPC is the largest and richest student startup competition in the world, and the competition had over 350 judges providing feedback to the teams, with hundreds of investors in attendance and over 700 people at the gala.
This isn’t the first time Baca and Harders have successfully joined forces to pitch for the company. A year ago, the duo brought home the $20,000 third-place award at the 2023 DOE EnergyTech University Prize competition in Austin, TX. “Kalin and I have worked together really closely for the last few years, both at KU and Icorium, and we make a great team,” says Harders. “Some of the Q&A sessions felt a little intense, but we received a lot of excellent and valuable feedback from the judges during and after the competition, and also a lot of supportive advice from investors afterwards that will help us strengthen the pitch and company’s strategy even more.” Harders, who will complete her Ph.D. in Chemical Engineering in July, will join Icorium full-time after graduation along with another KU engineering student, Luke Wallisch, who is an Icorium R&D Engineering Intern and KU Mechanical Engineering senior. “We feel very fortunate and are incredibly grateful to RBPC and all the judges and investors at the competition,” says Harders. “The plan has always been for me and Luke to join the company full-time when we graduate this summer. Thanks to the prizes and investment from the competition, we know for sure we can make that happen and can just focus on finishing strong at KU.
About Icorium
Icorium Engineering Company is a sustainable engineering company a spin-out of the University of Kansas’ Wonderful Institute for Sustainable Engineering (WISE-KU). Located at KU Innovation Park in Lawrence, KS, Icorium is developing novel technologies and transforming them into commercial solutions to enable and incentivize circular, sustainable economies for refrigerants and other critical materials. Learn more at www.icoriumengineering.com.
About the RBPC and Rice Alliance
The Rice Business Plan Competition, entering its 24th year, gives collegiate entrepreneurs real-world experience to pitch their startups, enhance their business strategy and learn what it takes to launch a successful company. Hosted and organized by the Rice Alliance for Technology and Entrepreneurship—which is Rice University’s internationally-recognized initiative devoted to the support of entrepreneurship—and Rice Business. Over 23 years it has grown from nine teams competing for $10,000 in prize money in 2001, to 42 teams from around the world competing for more than $1.5 million in cash and prizes. It is the largest and richest student startup competition in the world. The results of the 2024 competition can be found at 2024 Results | Rice Business Plan Competition.
About WISE-KU
The Wonderful Institute for Sustainable Engineering is advancing global sustainability through transformational engineering, science, and education. WISE-KU aims to focus on creating solutions that can be applied to real world issues promoting the societal, economic, and environmental benefits of sustainable and green engineering. The institute goals include: providing multidisciplinary scientific and technical expertise in applied sustainability research, developing sustainability concepts in engineering education for graduate and undergraduate students, working with industry to advance research and development in sustainability projects, creating measurement tools and frameworks for guiding the design of more sustainable products and processes, and developing education programs and certificates for industry and society that include the roles of engineering and science in creating a more sustainable future. To learn more about the Wonderful Institute for Sustainable Engineering, its faculty, students, and programs, please visit www.wise.ku.edu.
-Published on: 04/09/2024
Shiflett Research Members win awards at University of Kansas – 2024 Research Showcase

Evanna Dominic and Morela Chapman won 2nd and 3rd place in the Undergraduate Student Poster Competition, respectively, and Julia won 3rd place in the Graduate Student Poster Competition.
Congratulations Julia, Evanna, and Morela!
-Published on: 04/08/2024
University of Kansas Renames Institute for Sustainable Engineering after The Wonderful Company

Recognition Honors University’s Partnership with the Global Agriculture Company, co-founded and led by Stewart and Lynda Resnick
The University of Kansas’ Institute for Sustainable Engineering has a new name—Wonderful Institute for Sustainable Engineering-KU (WISE-KU). The naming builds on the university’s deep relationship with The Wonderful Company, a global agricultural company co-founded and led by Stewart and Lynda Resnick. The Wonderful Company is one of the largest privately held companies in the U.S. whose iconic brands include Wonderful Pistachios, POM Wonderful, FIJI Water, JUSTIN Wines, and more. Along with the renaming comes a $5 million commitment to enable the Institute to promote sustainable engineering initiatives.
“This substantial commitment by The Wonderful Company and Stewart and Lynda Resnick brings together researchers and students from multiple disciplines with industry partners to advance global sustainability through transformational engineering, science, and entrepreneurship,” said Foundation Distinguished Professor Mark B. Shiflett, Founder and Director of the Institute. “We are honored to name our Institute after our partners at Wonderful for their major investment in our Engineering students and university, as well as our ongoing collaboration to create solutions to today’s real-world issues promoting the societal, economic, and environmental benefits of sustainable engineering.”
Assistant Professor Ana Rita C. Morais in the Department of Chemical & Petroleum Engineering and Deputy Director of the Institute stated, “This convergent, inclusive approach fosters and supports innovation resulting in developing, inventing, and patenting novel processes and products that sustainably utilize food, water, and energy by recycling valuable resources while reducing our impact on the environment and protecting our planet.”
“Complex problems require new and novel approaches in order to arrive at workable solutions,” said Douglas A. Girod, Chancellor, University of Kansas. “The Resnicks are prime examples of how creative use of philanthropy can drive university research and discoveries. We’re grateful they’ve chosen to work with our talented faculty and students to solve some of today’s thorniest issues. Their investment, combined with the opportunity to work on real-world challenges, will benefit generations to come.”
The Resnicks have a long history of supporting leading research universities in driving research solutions to solve the world’s most critical environmental challenges across energy, water, food, and the climate. To date, the Resnicks, along with their foundations and The Wonderful Company, have invested nearly $2.6 billion in philanthropy and corporate social responsibility investments globally—in education, wellness, housing, and the arts–with more than $850 million pledged to universities for research and technologies around sustainability.
“Environmental sustainability must be one of the priorities for our planet and is a primary focus of our company’s operations. Succeeding in our efforts to care for our world requires research and innovation – everything from renewable energy and responsible water usage to rethinking pistachio waste,” said Eric Johnson, senior vice president of Capital Projects at The Wonderful Company and proud alumnus of the University of Kansas. The “Wonderful Institute for Sustainable Engineering at KU has taken a novel approach towards exploring new technologies and creating cutting-edge outputs that align with Wonderful’s mission to make our world a safer, healthier, and better home for generations to come.”
In the last five years, The Wonderful Company, which is one of the world’s largest nut processors, has worked in collaboration with WISE-KU researchers to find ways to repurpose 50 million pounds of pistachio shells, which until now went to carbon neutral fuel outlets or accumulated in piles on fallowed farmland. Researchers have found multiple ways to use them, including as an ingredient in animal feed.
About Wonderful Institute for Sustainable Engineering at the University of Kansas
Wonderful Institute for Sustainable Engineering (WISE) is advancing global sustainability through transformational engineering, science, and education. WISE aims to focus on creating solutions that can be applied to real world issues promoting the societal, economic, and environmental benefits of sustainable and green engineering. The institute goals include: providing multidisciplinary scientific and technical expertise in applied sustainability research, developing sustainability concepts in engineering education for graduate and undergraduate students, working with industry to advance research and development in sustainability projects, creating measurement tools and frameworks for guiding the design of more sustainable products and processes, and developing education programs and certificates for industry and society that include the roles of engineering and science in creating a more sustainable future. To learn more about Wonderful Institute for Sustainable Engineering, its faculty, students, and programs, please visit https://wise.ku.edu.
About The Wonderful Company
The Wonderful Company is a privately held $5 billion global company dedicated to harvesting health and happiness around the world. Its iconic brands include FIJI Water, POM Wonderful, Wonderful Pistachios, Wonderful Halos, Wonderful Seedless Lemons, Teleflora, and JUSTIN, Landmark, and Lewis Cellars wines.
To date, the Resnicks through their foundation, and The Wonderful Company have invested more than $2.5 billion in philanthropy, with more than $1.3 billion invested in environmental sustainability, to help combat climate change and preserve the planet for future generations. As longtime supporters of research and development, the Resnicks aim to create long-term global value through their gifts and open new figurative portals to sustainability in research, education, and society.
To learn more about The Wonderful Company, its products, and its core values, please visit https://wonderful.com, or follow The Wonderful Company on LinkedIn, Facebook, and Instagram. To learn more about the company’s corporate social responsibility impact, visit https://csr.wonderful.com.
-Published on: 02/12/2024
Hannah Uhl receives The Engineering Impact – Bobbie Banaszak Gleiter Scholarship

Hannah was chosen as the sole recipient of The Engineering Impact – Bobbie Banaszak Gleiter Scholarship, a Chi Omega Sorority National Scholarship. Bobbie Banaszak Gleiter is an initiate of Chi Beta Chapter at Purdue University, an accomplished aerospace engineer, and an advocate for women in engineering. She established the Engineering Impact – Bobbie Banaszak Gleiter Scholarship fund for Chi Omega collegians and alumnae pursuing a career in engineering.
Congratulations, Hannah!
-Published on: 12/04/2023
Future Leaders of Chemical Engineering


Hannah Uhl participated in the prestigious “Future Leaders of Chemical Engineering” 2-day workshop with all expenses paid at N.C. State University on Oct 22-23, 2023. Hannah presented her research on “Separations on Azeotropic Refrigerants using Ionic Liquid-Based Membranes”. Hannah works with PhD graduate student, Abby Harders.
Congratulations Hannah!
-Published on: 10/26/2023
Congratulations to Abby Harders and her team for two publications that were featured on the cover of Industrial Engineering Chemistry Research


Separation of Refrigerant Gases Using a Copolymer of Perfluoro(2,2-dimethyl-1,3-dioxole) (PDD) and Vinyl Acetate (VA)
By Abby N. Harders, Sarah Dixon, Brock Hines, Michael Lundin, Whitney White, and Mark B. Shiflett
Ind. Eng. Chem. Res. 2023, 62(37), 15148-15156, DOI: 10.1021/acs.iecr.3c01822
Artist Acknowledgment: Gil J. Ortiz
Solubility, Diffusivity, and Permeability of HFC-32 and HFC-125 in Amorphous Copolymers of Perfluoro(butenyl vinyl ether) and Perfluoro(2,2-dimethyl-1,3-dioxole)
By Abby N. Harders, Erin R. Sturd, Luke Wallisch, Hannes Schmidt, Yuniva Mendoza-Apodaca, David R. Corbin, Whitney White, Christopher P. Junk, and Mark B. Shiflett
Ind. Eng. Chem. Res. 2023, 62(9), 4054-4063, DOI: 10.1021/acs.iecr.2c04518.
Artist Acknowledgment: Gil J. Ortiz
-Published on: 10/13/2023
Ionic Liquid Selection for the Separation of Refrigerant Mixtures Using Extractive Distillation

Max Cordry and Tessie May, Senior Undergraduates in Chemical Engineering, pin their latest publication with coauthors Ethan Finberg (MS, 2022) and Dr. Kalin Baca (PhD, 2023) entitled “Ionic Liquid Selection for the Separation of Refrigerant Mixtures Using Extractive Distillation” that recently published in Industrial Engineering Chemistry Research. Congratulations everyone!
-Published on: 10/04/2023
Kalin Baca, PhD Chemical Engineering

Dr. Kalin R. Baca completed her PhD and graduated with Honors. Her thesis was entitled “Investigation of Ionic Liquids for the Separation of Azeotropic Hydrofluorocarbon Refrigerant R-410A”. She is cofounder of Iconium Engineering Company located at KU Innovation Park that is commercializing ionic liquids for separation of refrigerant mixtures.
Congratulations Dr. Baca!
-Published on: 10/04/2023
Prof. Mark Shiflett awarded KU’s highest research achievement award

Congratulations! Prof. Mark Shiflett was awarded the 2023 Higuchi – Irvin Youngberg Research Award in Applied Sciences for his work to develop sustainable processes and products for the chemical industry. The Higuchi Research Achievement Awards are KU’s highest award for faculty accomplishments in research, scholarship, and creative activity. The awards are designed to recognize significant research achievement conducted at the University of Kansas or any other Regents institution.
-Published on: 4/30/2023
DOE EnergyTech University National Competition

Grad students Kalin Baca and Abby Harders participated in a competition put on by the Department of Energy. They won 3rd place for their presentation about utilizing membrane technology and extractive distillation to separate and recycle complex refrigerant mixtures at the end of their life. 184 teams from 124 schools across 44 states participated in the EnergyTech competition. Read more about the competition here. Well done, Kalin and Abby!
-Published on: 4/11/2023
Goodbye, Dr. Rajkumar Kore!

We celebrated Dr. Rajkumar Kore who has worked five years with Prof. Shiflett’s research group and wish him well in his new position at INVISTA.
-Published on: 3/18/2023
Separation of Refrigerant Mixtures using Membranes

PhD student Abby Harders recently published a new paper:
Solubility, Diffusivity, and Permeability of HFC-32 and HFC-125 in Amorphous Copolymers of Perfluoro(butenyl vinyl ether) and Perfluoro(2,2-dimethyl-1,3-dioxole)
Authors: Abby N. Harders, Erin R. Sturd, Luke Wallisch, Hannes Schmidt, Yuniva Mendoza-Apodaca, David R. Corbin, Whitney White, Christopher P. Junk and Mark B. Shiflett
-Published on: 2-14-2022
2022 AIChE Poster Awards

Claire Sabolay – 1st Place | Environmental Group 1 & 3rd Place | Environmental Division
Dorothy Haggard – 2nd Place Separations Group 1
Lucia Matamoros Valenciano – 3rd Place | Separations Group 1
Congratulations to Claire, Dorothy, and Lucia!
-Published on: 12/06/2022
2022 AIChe Conference



- AIChE Poster Session 11-15-2022 “Prof. Aaron Scurto, Karim Al-Barghouti, Kalin Baca, Andrew Yancey, Abby Harders, and Prof. Mark Shiflett at AIChE Phoenix Poster Session, 11-15-2022”
- Abby Mark AIChE 11-14-2022 “Abby Harders and Prof. Mark Shiflett at AIChE RAPID poster session, Phoenix AZ, 11-15-2022”
- AIChE EFRI In-person Meeting 11-16-2022 “NSF EFRI Research Team at AIChE Phoenix 2022 meeting, 11-16-2022”
-Published on: 11/30/2022
Chemical & Petroleum Engineering Awards Banquet
Several of our group members were honored at the departmental awards banquet.









Nicole Montoya (top left) – Outstanding Master’s Chemical Engineering Research Award
Madelyn Bennett (top center) – Outstanding Academic Achievement Award (GPA 3.95 or higher), Academic Honors Award (GPA 3.5 or higher)
Lucia Matamoros Valenciano (top right) – Academic Honors Award (GPA 3.5 or higher)
Daniel Cho (middle row left) – Academic Honors Award (GPA 3.5 or higher)
Max Cordry (middle row center)– Fred Kurata Thermodynamics Award
Alex Henne (middle row right), Sophia Terian (bottom left), Nathan Ohl, and Melvin Loo – Best Senior Chemical Engineering Laboratory Group
Sophia Terian (bottom left) – Academic Honors Award (GPA 3.5 or higher)
Hannah Uhl (bottom center) – Outstanding Junior in Chemical Engineering
Sarah Dixon (bottom right) – Outstanding Sophomore in Chemical Engineering
Congratulations, Raj!

Congratulations to Dr. Raj Kore and his wife Vidya who had a baby boy, named Virajas, born on Monday, Nov 7, 2022!
-Published on: 11/11/2022
3 Minute Thesis

Ms. Kalin Baca, PhD student, Chemical Engineering, took 2nd Place Overall in the University of Kansas, 3 Minute Thesis Competition. The title of her presentation was “Recycling Refrigerants to Reduce Global Warming”.
Way to go Kalin!
-Published on: 11/11/2022
Fall 2022 AIChe Conference
Sunday, Nov 13 – Friday, Nov 18, 2022
Phoenix Convention Center
Phoenix, Arizona
The Shiflett Research Group has 15 presentations and posters covering a variety of research topics from refrigerant separations to PFAS removal to undergraduate laboratory education.
| Presenter | Title | Date | Time | Location | Format |
|---|---|---|---|---|---|
| Felipe Anaya | A State-of-the-Art Pilot-Scale Distillation Column for the Unit Operations Laboratory at the University of Kansas | 15-Nov | 4:32-4:50 | W-105B | Oral |
| Felipe Anaya | A Novel Approach for Laboratory Experiments in Process Dynamics and Control | 15-Nov | 6:02-6:20 | W-105B | Oral |
| Ethan Finber/MarkShiflett | Separation of Azeotropic Refrigerant Mixtures Using Extractive Distillation with Ionic Liquid Entrainers | 14-Nov | 4:48-5:06 | N-132A | Oral |
| Madelyn Bennett | Chlorine Stability of Bio-Inspired Liquid Infused Membranes | 14-Nov | 11:00-1:30 | North Hall E | Poster |
| Kalin Baca | Phase Equilibria for HFC-32, HFC-125, and Binary Mixtures (R-410A) with a Variety of Ionic Liquids | 15-Nov | 4:30-6:00 | North Hall E | Poster |
| Andrew Yancey | Phase Equilibria for HFC-32, HFC-125, and Binary Mixtures (R-410A) with a Variety of Ionic Liquids | 15-Nov | 4:30-6:00 | North Hall E | Poster |
| Karim Al-Barghouti | Phase Equilibrium and Transport Properties of Water and Ionic Liquids Used in Biomass Processing | 14-Nov | 10:20-10:40 | N-223 | Oral |
| Clarice Sabolay | Removing Pfas from Water Using Zeolites: Cheaper and More Efficient | 14-Nov | 11:00-1:30 | North Hall E | Poster |
| Kalin Baca | Project Earth (Environmentally Applied Research Towards Hydrofluorocarbons) Ionic Liquids Approach | 15-Nov | 4:30-6:00 | North Hall E | Poster |
| Abby Harders | Separation of HFC-32, HFC-125, HFC-134a, HCFC-22, and HFO-1234yf Using Copolymers of Perfluoro(butenyl vinyl ether) and Perfluoro(2,2-dimethyl-1,3-dioxole) | 15-Nov | 4:30-6:00 | North Hall E | Poster |
| Dorothy Haggard | Investigation of Imidazolium-Based Ionic Liquids with the Sorption of HFC-32 and HFC-125 Refrigerant Gases | 14-Nov | 11:00-1:30 | North Hall E | Oral |
| Lucia Valenciano | Measuring Heat of Absorption for Hydrofluorocarbon Refrigerants and Ionic Liquids | 14-Nov | 11:00-1:30 | North Hall E | Poster |
| Charles Ponge | Adsorption of Pfas By Zeolites | 14-Nov | 6:35-7:00 | N-225A | Oral |
| Ethan Finberg/Mark Shiflett | Separation of Refrigerant Mixtures Using Extractive Distillation with Ionic Liquid Entrainers | 15-Nov | 4:30-6:00 | North Hall E | Poster |
| Andrew Yancey | The Use of Zeolites and Carbons for the Separation of Refrigerant R-410A: Thermodynamic Modeling of Pure Gas and Binary Sorption | 16-Nov | 3:36-3:54 | N-131C | Oral |
| KU Evening Reception | 13-Nov | 8:15-10:15 | N-121AB | KU Evening Reception |
-Published on: 10/17/2022
Separation of Refrigerant Mixtures

Our graduate student, Andrew Yancey-Jardon, recently published a new paper:
Separation of Azeotropic Hydrofluorocarbon Refrigerant Mixtures: Thermodynamic and Kinetic Modeling for Binary Adsorption of HFC-32 and HFC-125 on Zeolite 5A
Authors: Andrew D. Yancey, Dr. Darren P. Broom, Mark G. Roper, Dr. Michael J. Benham, Dr. David R. Corbin, Prof. Mark B. Shiflett
-Published on: 9/05/2022
Ionic Liquids Research Posters


Kalin Baca and Dorothy Haggard both presented Research Posters at the “2022 Gordon Research Conference on Ionic Liquids” that was held in Newry, Maine, Aug 7 – 12, 2022. Kalin received recognition and an award for one of the top-4 posters out of 85 posters presented at the conference
-Published on: 8/14/2022
Ethan Finberg, M.S. Chemical Engineering

Congratulations to Ethan Finberg who successfully defended his M.S. Chemical Engineering Thesis entitled “Refrigerant Separation with Extractive Distillation using Ionic Liquid Entrainers”.
-Published: 6/03/2022
New Refrigerant in Watson Library

University of Kansas testing new environmentally friendly refrigerant in Watson Library. Learn more here.
-Published: 5/16/2022
Nicole Montoya, M.S. Chemical Engineering

Congratulations to Nicole Montoya who successfully defended her M.S. Chemical Engineering Thesis entitled “Protein Thermal Stabilization and Delivery via Adsorption onto Porous Silicas”.
-Published: 5/11/2022
Top Scientists Worldwide

Prof. Mark B. Shiflett, Foundation Distinguished Professor, is among the top scientists worldwide. Dr. Shiflett was selected as one of the Top 2% of Scientists Worldwide from over 9 million scientists. The list is compiled by experts at Stanford University, based on number of publication citations. Congratulations, Dr. Shiflett!
-Published: 4/29/2022
2022 Chemical Engineering Departmental Awards
Recently the University of Kansas’ Department of Chemical Engineering held its annual awards ceremony. Three of our members were honored at the ceremony. Senior Greta Olsen won Outstanding Undergraduate Researcher. Erin Sturd won Most Outstanding Senior. Nicholas Reding won the Frank Bowdish Outstanding PhD award. Join us in congratulating Greta Olsen, Erin Sturd, and Nicholas Reding for their accomplishments!
-Published on: 4/26/2022
Tour of KU Natural History Museum

The Shiflett Research group recently visited the University of Kansas Natural History Museum. The museum contains a variety of exhibits, such as the Panorama shown here, which contains animal mounts dating back to 1893.
-Published on: 12/17/2021
2021 Graduation!

This past weekend was the graduation ceremony for Ankit Verma. Congratulations, Dr. Verma on successfully obtaining your PhD in Chemical Engineering!
-Published on: 12/17/2021
Congratulations, Nick and Ankit!
Congratulations to Nick Reding and Ankit Verma for graduating this semester and completing their PhDs! Shown here are photos from our celebration of Nick and Ankit’s time at KU.
-Published on: 11/29/2021
REU Presentations
This summer, five REU students joined the Shiflett Research Group to gain hands on experience in a variety of research areas. The photos above show the students giving presentations over their research projects.
-Published on: 5/31/2021
2021 Summer Picnic
The Shiflett, Allgeier, Scurto, and Morais Research Groups recently came together for a summer picnic and enjoyed food, games, and community.
-Published on: 7/19/2021
On the Run!

The Shiflett and Allgeier Research group recently participated in the GEAK 5K run! Ph.D candidate Ankit Verma placed 3rd overall in the men’s division of the race!
-Published on: 5/31/2021
Journal of Ionic Liquids

Dr. Shiflett has been selected by Elsevier to be the first Editor-In-Chief of the newly launched journal entitled “Journal of Ionic Liquids”. A link to the Journal of Ionic Liquids can be found here: https://www.journals.elsevier.com/journal-of-ionic-liquids.
The journal will publish papers on the broad field of ionic liquids that include topics such as thermophysical properties, coatings, catalysis and chemical reactions, interfacial phenomena, lubricants, materials, membranes, molecular modeling, polymers, separations, scale-up, toxicity, and more.
The Journal of Ionic Liquids will be the first journal dedicated to the exciting and expanding field of ionic liquids.
-Published on: 5/19/2021
Congrats, Dr. Shiflett!

Dr. Shiflett recently received the 2021 University of Kansas Gould Award in Advising. The Gould Award is offered to an outstanding advisor nominated by students in the School of Engineering. Congratulations Dr. Shiflett!
-Published on: 5/19/2021
Congratulations, Madelyn!

Madelyn Bennet, an undergraduate researcher who works under Kalin Baca in Project EARTH, was chosen as the University of Kansas “Most Outstanding Sophomore in Chemical Engineering”. Congratulations, Madelyn!
Congratulations, Raj!

Dr. Raj Kore, a post doctoral researcher in the Shiflett Research Group, recently became a father to Rajvi, the newest little jayhawk in our group! Congratulations Raj!
Greetings from the Shiflett Research Group

The Shiflett Research Group recently took a photoshoot to celebrate the unique gifts that each one of our member brings to our group. T-shirts were made for all of our members with a word of their choice. We are happy to have such a unique group of people contributing to science and the community at the University of Kansas.
New Book Chapter!

A chapter entitled “A Sustainable Oxalate Process for Recovery of Metals from LiCoO2: Experimental and Modeling Study” by PhD student Ankit Verma, Dr. David Corbin and Dr. Mark Shiflett has been published in the “Ni-Co 2021: The 5th International Symposium on Nickel and Cobalt.” To learn more, follow the link below:
https://www.springer.com/gp/book/9783030656461
-Published on: 4/07/2021
Congrats, Erin!

Erin Sturd, a researcher in the membrane program of Project EARTH, was recently awarded the Undergraduate Research Award (UGRA) for the Spring 2021 semester. Erin is working on the development of mixed matrix membranes for the selective separation of hydrofluorocarbon refrigerants. Congratulations, Erin!
-Published on: 3/30/2021
New Publication!

A paper entitled “Effect of Particle Morphology on Metal Dust Explosion Severity and Sensitivity” by co-authors Nicholas Reding and Ankit Verma has been published in the Journal of Loss Prevention in the Process Industries. To learn more about this paper, follow the link below!
https://doi.org/10.1016/j.jlp.2021.104396
-Published on: 3/30/2021
Congratulations to our undergraduate researchers!
Madelyn Bennett and Lucia Matamoros were selected as SELF Engineering Fellows for the 2023 cohort. SELF fellows will develop their communication, business, engineering, leadership, managerial, entrepreneurial and interpersonal skills with an enrichment program. Additionally they will receive the SELF Undergraduate Scholarship. Greta Olsen won the Gerald Lage award, which implies: lettering in athletics, completing 100 credit hours, and having abound a 3.8 GPA. It is the highest academic honor given by the Big 12 Conference to student athletes. Madelyn, Lucia and Greta are all members of Project EARTH’s ionic liquids team. Congratulations!
-Published on: 3/30/2021
Congratulations, Ankit!

Ankit Verma received the 1st place KU presentation award at the Capitol Graduate Research Summit held virtually this year. This summit provides an opportunity for selected graduate students to expose state government, education officials, and the public to the quality of graduate research performed in the state of Kansas. Ankit’s research focuses on the development of sustainable processes for recovery of critical metals using oxalate chemistry and in this summit, he presented his work on the sustainable process for recycling of spent lithium-ion batteries. For more information, see the following link: https://graduate.ku.edu/2021-cgrs-presenters
-Published on: 1/24/2021
Koerner Family Foundation Supplemental Stipend Award

Congratulations to Nick Reding on being selected to receive a $10K supplemental stipend award from the Koerner Family Foundation! The Koerner Family Foundation aspires to promote growth for engineers working to uncover innovative solutions to future challenges. Funding from this foundation will act as direct support for continued research on metal dust combustion dynamics and potential explosion protection methods.
-Published on: 1/14/2021

We are still accepting applications for the 2021 REU program in the Chemical and Petroleum Engineering department at the University of Kansas. Take advantage of this opportunity to learn about porous materials and grow as a researcher and scientist! The program will take place from May 24-July 30, 2021. The application deadline is January 25th, 2021. Apply at https://cpe.ku.edu/reu-program
-Published on: 1/12/2021
Project EARTH featured on DOE Homepage!

Project EARTH, a collaborative project focused on developing environmentally friendly methods of separating refrigerant mixtures that contribute to global warming, has been featured on the DOE Office of Science homepage! Click the following link to view our post!
-Published on: 12/12/2020
Congratulations, Nicole!

Project SAVE just published their first paper and made it to the cover of Langmuir! In this publication Nicole Montoya and other members of Project SAVE discuss how to adsorb proteins into mesoporous silica to conserve protein structure and activity. Project SAVE is eager to build upon this work and begin adsorbing the influenza vaccine and one of the key proteins in the COVID-19 protein based vaccine.
-Published on: 12/12/2020
New Publication!

A new publication entitled “Thermochemical Insights into Stability and Hydration of Ion-Exchanged Zeolite ZK-5 (KFI Framework)” was published in “The Journal of Physical Chemistry C”. Ankit Verma, Dr. David Corbin, and Dr. Mark Shiflett co-authored this paper in collaboration with Dr. Navrotsky’s group at Arizona State University. This thermochemical study demonstrates the critical role of extra-framework cations and documents the complexity of ion exchange effects in zeolites with complicated topology and cation distribution. To learn more about this paper, click on the following link: doi=10.1021/acs.jpcc.0c06796&ref=pdf
-Published on: 12/01/2020
Project EARTH Featured in KU Discoveries

Project EARTH (Environmentally Applied Research Towards Hydrofluorocarbons) was recently featured in “KU Discoveries” online magazine. Project EARTH is focused on how to effectively recycle and repurpose hydrofluorocarbon (HFC) refrigerants to reduce their impact on global warming and climate change. The project is supported by a four-year, $2 million grant from the National Science Foundation and is a collaboration between the University of Kansas, University of Notre Dame, Texas A&M University, and Rutgers University. In addition, Oak Ridge National Laboratory (ORNL), Brookhaven National Laboratory (BNL), the National Institute of Standards and Technology (NIST), and several private companies are working closely with the Project EARTH team. To read the full article, click the link below:
-Published on: 12/01/2020
Join the Shiflett Research Group at the 2020 AIChE Meeting!

The 2020 Virtual AIChe Meeting is this week! The Shiflett Research Group has 18 presentations and posters covering a variety of different research topics and interests. If you are interested in attending any of the conference talks or poster sessions, here are helpful resources to help you navigate this year’s virtual conference:
-Published on: 11/16/2020
A Novel Chemical Engineering Laboratory Model at the University of Kansas

Ethan Finberg, along with David Griffin and Mark Shiflett, recently published a paper on the KU Senior Chemical Engineering Lab course in the journal, Chemical Engineering Education. This publication explains how this unique course structure is able to enroll up to 92 students and teach three experiments with safety tours, presentations, and reports over the duration of a semester. This publication also discusses the experimental apparatuses used to produce accurate and repeatable results. All apparatuses are portable to efficiently utilize the limited lab space or be displayed in the classroom for teaching purposes. Congratulations Ethan!
-Published on: 11/10/2020
New Industry Collaboration

A $1 million, 18-month collaboration between the University of Kansas School of Engineering and the RAPID Manufacturing Institute for Process Intensification launched in 2017 by the American Institute of Chemical Engineers will develop technology to separate gas using renewable, high-performance furanic-based polymers that were originally developed for replacing PET-based soda bottles. Click the above link to read more about this exciting project!
-Published on: 10/15/2020
Join us at our monthly safety meetings!
 The Chemical and Petroleum Engineering Department (C&PE) is holding monthly safety meetings on the 3rd Wednesday of each month from 9:00 – 9:45 a.m. in the Breen Conference Center (ground floor of Slawson Hall). The meetings are also available via Zoom. Anyone is welcome to attend. Interested in presenting a safety topic at an upcoming meeting? Contact Dr. Berlyn Mellein (berlyn.mellein@ku.edu). Follow us on Facebook, Instagram, and Twitter to get updates about upcoming meetings!
The Chemical and Petroleum Engineering Department (C&PE) is holding monthly safety meetings on the 3rd Wednesday of each month from 9:00 – 9:45 a.m. in the Breen Conference Center (ground floor of Slawson Hall). The meetings are also available via Zoom. Anyone is welcome to attend. Interested in presenting a safety topic at an upcoming meeting? Contact Dr. Berlyn Mellein (berlyn.mellein@ku.edu). Follow us on Facebook, Instagram, and Twitter to get updates about upcoming meetings!
-Published on: 10/10/2020
Congratulations Kalin, Abby, Ana, and Greta!
 A new publication entitled “Phase Equilibria, Diffusivities, and Equation of State Modeling of HFC-32 and HFC-125 in Imidazolium-Based Ionic Liquids for the Separation of R-410A” authored by graduate students Abby Harders and Kalin Baca, former post-doctoral researcher Ana Morais, and undergraduate researcher Greta Olsen was recently published in the Journal of Industrial and Engineering Chemistry Research. This paper was a collaborative effort between Project EARTH researchers in the Shiflett Research Group and Dr. Edward Maginn’s research group at the University of Notre Dame. Click the following link to learn more!
A new publication entitled “Phase Equilibria, Diffusivities, and Equation of State Modeling of HFC-32 and HFC-125 in Imidazolium-Based Ionic Liquids for the Separation of R-410A” authored by graduate students Abby Harders and Kalin Baca, former post-doctoral researcher Ana Morais, and undergraduate researcher Greta Olsen was recently published in the Journal of Industrial and Engineering Chemistry Research. This paper was a collaborative effort between Project EARTH researchers in the Shiflett Research Group and Dr. Edward Maginn’s research group at the University of Notre Dame. Click the following link to learn more!
doi.org/10.1021/acs.iecr.0c02820
-Published on: 9/19/2020
Congratulations, Ankit!
Congratulations to Ankit Verma, co-inventor on International Patent WO 2020/086509A1 that was recently published. The patent claims cover the use of oxalate-based reagents for the recovery and separation of Li and Co from used lithium-ion battery (LIB) cathodes. Cobalt in particular is a critical metal with limited supply and methods for the economical recycle of Li and Co will be important in the future as the LIB market rapidly expands
-Published on: 9/19/2020
New Publication!
A paper entitled “Power generation from waste heat: Ionic liquid‐based absorption cycle versus organic Rankine cycle” where Dr. Shiflett was one of the authors has been published in the AIChE Journal. To learn more about this paper, click the link below!
-Published on: 9/16/2020
Grant Announcement
Prof. Mark Shiflett and Prof. Aaron Scurto were recently awarded a $2,000,000 Emerging Frontiers in Research and Innovation (EFRI) grant to study the separation and recycling of high global warming potential (GWP) refrigerant mixtures. Project EARTH (Environmentally Applied Research Towards Hydrofluorocarbon) will use a combination of experiments, advanced computer simulations, and rigorous analytical methods to discover, synthesize, and test ionic liquids that are efficient at separating azeotropic hydrofluorocarbon (HFC) mixtures. The research will be conducted by a team of researchers located at the University of Kansas (lead institution), the University of Notre Dame, Texas A&M University, and Rutgers University in collaboration with Brookhaven National Lab, Oak Ridge National Lab and the National Institute of Standards and Technology along with two industry partners (Chemours and Iolitec). The technical, economic, and environmental impacts of Project EARTH is immense, given the millions of kilograms of high GWP refrigerants that must be removed from the market. The market for recycling refrigerants is valued at more than a billion dollars and preventing the release of high-GWP refrigerants into the Earth’s atmosphere is equivalent to eliminating 175 million metric tons of CO2 (or annual emissions from 50 million cars).
-Published on: 9/01/2020
Congrats, Rajkumar!
 A review paper by Dr. Rajkumar Kore, a post-doctoral researcher in the Shiflett Research Group, recently got accepted in the Journal of Industrial & Engineering Chemistry Research. His paper is entitled “Review of Isobutane Alkylation Technology Using Ionic Liquid-Based Catalysts – Where Do We Stand?” Click below to read more!
A review paper by Dr. Rajkumar Kore, a post-doctoral researcher in the Shiflett Research Group, recently got accepted in the Journal of Industrial & Engineering Chemistry Research. His paper is entitled “Review of Isobutane Alkylation Technology Using Ionic Liquid-Based Catalysts – Where Do We Stand?” Click below to read more!
-Published on: 8/16/2020
New Publication!
 A paper entitled “A Review of Porous Adsorbents for the Separation of Nitrogen from Natural Gas, Industrial Engineering Chemistry Research” authored by former post-doctoral researcher Sachin U. Nandanwar, senior scientist David R. Corbin, and Dr. Shiflett was recently published in Industrial & Engineering Chemistry Research. Click the following link to read more!
A paper entitled “A Review of Porous Adsorbents for the Separation of Nitrogen from Natural Gas, Industrial Engineering Chemistry Research” authored by former post-doctoral researcher Sachin U. Nandanwar, senior scientist David R. Corbin, and Dr. Shiflett was recently published in Industrial & Engineering Chemistry Research. Click the following link to read more!
-Published on: 7/26/2020
 Congratulations, Grad!
Congratulations, Grad!
Abby Harders, a student who joined the Shiflett Research Group as a researcher in the 2019 KU Chemical Engineering REU Program, graduated from Bethel College this past weekend. Abby graduated Summa Cum Laude with a 4.0 GPA and earned a BA in Chemistry and Mathematics. Abby was recently named a University of Kansas Chancellor’s Fellow and will begin her doctorate research this summer. Join us in congratulating Abby for graduating from Bethel College with the highest of honors! To view the Bethel College Virtual Commencement, including 30-second videos by Abby and the other Bethel graduates, click the link below!
https://www.bethelks.edu/success/graduation
-Published on: 5/18/2020
Congratulations Nick!
 Nick Reding has successfully completed his PhD Comprehensive Exam. Nick’s proposal was titled “Design of Effective Energy Storage System Ventilation Techniques: Correlating Initial Turbulence to Hydrogen Deflagration Severity”. Join us in congratulating Nick for his success in completing his Comprehensive Exam!
Nick Reding has successfully completed his PhD Comprehensive Exam. Nick’s proposal was titled “Design of Effective Energy Storage System Ventilation Techniques: Correlating Initial Turbulence to Hydrogen Deflagration Severity”. Join us in congratulating Nick for his success in completing his Comprehensive Exam!
-Published on: 5/16/2020
Congratulations Tugba!
 Dr. Tugba Turnaoglu has been named the 2020 Recipient of the Frank Bowdish Outstanding PhD award at the University of Kansas. Tugba defended her thesis on “Phase Behavior of Binary Ionic Liquid Systems: Ionic Liquids with Ammonia, Carbon Dioxide, and Dihydroxy Alchohols” in December of 2019 and is currently working as a post-doctoral researcher at Oak Ridge National Laboratory. Join us in congratulating Tugba for receiving this prestigious award!
Dr. Tugba Turnaoglu has been named the 2020 Recipient of the Frank Bowdish Outstanding PhD award at the University of Kansas. Tugba defended her thesis on “Phase Behavior of Binary Ionic Liquid Systems: Ionic Liquids with Ammonia, Carbon Dioxide, and Dihydroxy Alchohols” in December of 2019 and is currently working as a post-doctoral researcher at Oak Ridge National Laboratory. Join us in congratulating Tugba for receiving this prestigious award!
-Published on: 5/16/2020
Congratulations Ana!
 Dr. Ana Morais, former Shiflett Research Group post doctoral researcher, recently published a paper in the AIChE Journal and a paper in Industrial and Engineering Chemistry Research. The first paper is titled “Phase equilibrium and diffusivities of hydrofluorocarbons in a synthetic polyol ester lubricant”. The second paper is titled “Solubility and Diffusivity of Hydrofluoroolefin Refrigerants in a Polyol Ester Lubricant”. Ana is now working at the National Renewable Energy Laboratory. Click the following links to read more about Ana’s papers:
Dr. Ana Morais, former Shiflett Research Group post doctoral researcher, recently published a paper in the AIChE Journal and a paper in Industrial and Engineering Chemistry Research. The first paper is titled “Phase equilibrium and diffusivities of hydrofluorocarbons in a synthetic polyol ester lubricant”. The second paper is titled “Solubility and Diffusivity of Hydrofluoroolefin Refrigerants in a Polyol Ester Lubricant”. Ana is now working at the National Renewable Energy Laboratory. Click the following links to read more about Ana’s papers:
doi.org/10.1021/acs.iecr.9b06821
-Published on: 5/08/2020
Congratulations Ankit!
 Graduate student Ankit Verma recently published a paper titled “Separation of Lithium and Cobalt from LiCoO2: A Unique Critical
Graduate student Ankit Verma recently published a paper titled “Separation of Lithium and Cobalt from LiCoO2: A Unique Critical
Metals Recovery Process Utilizing Oxalate Chemistry” in ACS Sustainable Chemistry & Engineering. Click the following link to learn more about Ankit’s work:
doi.org/10.1021/acssuschemeng.0c01128
-Published on: 5/08/2020
Congratulations David!
 Dr. David Minnick, former post doctoral research in the Shiflett Research Group, recently published a paper in the Journal of Chemical Engineering Data. David’s paper is entitled “Solubility and Diffusivity of Bromodifluoromethane (Halon-1201) in Imidazolium Ionic Liquids: [C2C1im][Tf2N], [C4C1im][BF4], and [C4C1im][PF6]”. David is now working as a Research Investigator at DuPont. Click the following link to read more about David’s paper:
Dr. David Minnick, former post doctoral research in the Shiflett Research Group, recently published a paper in the Journal of Chemical Engineering Data. David’s paper is entitled “Solubility and Diffusivity of Bromodifluoromethane (Halon-1201) in Imidazolium Ionic Liquids: [C2C1im][Tf2N], [C4C1im][BF4], and [C4C1im][PF6]”. David is now working as a Research Investigator at DuPont. Click the following link to read more about David’s paper:
doi.org/10.1021/acs.jced.0c00022
-Published on: 5/08/2020
Congratulations Nick!
 A paper by Nick Reding, graduate student in the Shiflett Research Group, recently got accepted in the Journal of Loss Prevention in the Process Industries. His paper is entitled “Consequence Prediction for Dust Explosions Involving Interconnected Vessels using Computational Fluid Dynamics Modeling”. Read more about Nick’s paper by clicking the following link:
A paper by Nick Reding, graduate student in the Shiflett Research Group, recently got accepted in the Journal of Loss Prevention in the Process Industries. His paper is entitled “Consequence Prediction for Dust Explosions Involving Interconnected Vessels using Computational Fluid Dynamics Modeling”. Read more about Nick’s paper by clicking the following link:
doi.org/10.1016/j.jlp.2020.104149
-Published on: 5/08/2020
Abby Harders named Chancellors Fellow
 Abby Harders has been selected to receive the University of Kansas Chancellors Doctoral Fellowship. The Chancellors Doctoral Fellowship is offered yearly to four incoming graduate students across all fields of study at KU who demonstrate the Chancellor’s values for doctoral education—exceptional academic ability and impressive research productivity. The Chancellors Fellowship was created by Chancellor Bernadette Gray-Little in 2013 and is being continued by current KU Chancellor, Doug Girod. Join us in congratulating Abby and the other Chancellors Doctoral Fellows!
Abby Harders has been selected to receive the University of Kansas Chancellors Doctoral Fellowship. The Chancellors Doctoral Fellowship is offered yearly to four incoming graduate students across all fields of study at KU who demonstrate the Chancellor’s values for doctoral education—exceptional academic ability and impressive research productivity. The Chancellors Fellowship was created by Chancellor Bernadette Gray-Little in 2013 and is being continued by current KU Chancellor, Doug Girod. Join us in congratulating Abby and the other Chancellors Doctoral Fellows!
To learn more about the Chancellors Doctoral Fellowship, see the following link:
https://graduate.ku.edu/chancellors-doctoral-fellowship
-Published on: 5/04/2020
Kalin Baca named SELF Graduate Fellow
 Kalin Baca was recently selected to receive the University of Kansas Madison and Lila SELF Graduate Fellowship. This fellowship is offered to exceptional graduate students who demonstrate the potential to positively contribute to their area of study and to society. In 1989, Madison and Lila Self created the Self Graduate Fellowship because of their “strong belief in the vital importance of developing leadership for tomorrow”. Join us in congratulating Kalin and 11 other doctoral students for being selected as a Self Graduate Fellow!
Kalin Baca was recently selected to receive the University of Kansas Madison and Lila SELF Graduate Fellowship. This fellowship is offered to exceptional graduate students who demonstrate the potential to positively contribute to their area of study and to society. In 1989, Madison and Lila Self created the Self Graduate Fellowship because of their “strong belief in the vital importance of developing leadership for tomorrow”. Join us in congratulating Kalin and 11 other doctoral students for being selected as a Self Graduate Fellow!
To learn more about the SELF Graduate fellowship and this year’s fellows, see the following link:
https://news.ku.edu/2020/04/30/ku-announces-new-2020-2024-self-graduate-fellows
-Published on: 5/04/2020
Kansas Undergraduate Research Symposium
Greta Olsen, sophomore chemical engineering major, was selected as one of only 5 undergraduates from the University of Kansas – Lawrence campus to present her research at the Kansas Undergraduate Research Symposium held at the Kansas State Capitol on March 4. Greta is working with a team of graduate students on Project EARTH – Environmentally Applied Research Towards Hydrofluorocarbons, which is developing new methods for separating azeotropic refrigerant mixtures so that they can be recycled, ultimately leading to a lower global warming impact.
-Published on: 3/04/2020
Frontiers Research Day
Nicole Montoya, Graduate student in Dr. Shiflett’s lab, presented a poster entitled “Mesoporous Silica-Immobilized Vaccines for Improving Thermo-tolerance and Shelf-life“ at the Frontiers Research Day at University of Kansas Edwards Campus. Nicole is leading a multi-disciplinary team composed of members from the School of Engineering, KU Structural Biology Center, and Pharmaceutical Chemistry focused on the development of mesoporous silicas to thermally stabilize vaccines so that refrigeration is no longer required
-Published on: 3/04/2020
Commercial Applications of Ionic Liquids
Dr. Shiflett recently published a book titled “Commercial Applications of Ionic Liquids“! Those interested in ordering the book can visit here for more information.

-Published on: 2/19/2020
On the Cover!
Dr. Turnaoglu’s published work, “High-Pressure Vapor-Liquid Equilibria of 1-Alkyl-1-Methylpyrrolidinium Bis(trifluoromethylsulfonyl)imide Ionic Liquids and CO2“, has been featured in the Journal of Chemical & Engineering Data.
-Published on: 1/1/2020
Congratulations, Tugba!

Tugba Turnaoglu successfully defended her Ph.D. Thesis entitled “Phase Behavior of Binary Ionic Liquid Systems: Ionic Liquids with Ammonia, Carbon Dioxide, and Dihydroxy Alcohols” and received Honors from her committee. Congratulations, Dr. Tugba Turnaoglu!
-Published on: 12/11/2019
Congratulations, Nick!
Nick Reding, a Graduate student in the Shiflett Research group, successfully defended his Masters thesis in Chemical Engineering on November 25th, 2019. His thesis is entitled “Mitigation of Metal Dust Deflagrations via Thermal Analysis and Active Explosion Suppression”. Congratulations Nick!

-Published on: 12/1/2019
On the Cover!
Nick Reding’s recently published work, “Mitigation of Iron and Aluminum Powder Deflagrations via Active Explosion Suppression in 1 m3 Sphere Vessel“, has recently been featured on a journal cover in Industrial & Engineering Chemistry Research (IECR). Congratulations!

-Published on: 12/1/2019
Nice work, Ankit!
Ankit Verma, PhD Candidate in Shiflett Research Group, won the first place in the Graduate Student Poster Competition for his poster entitled “Role of Oxalates in Metal Separation and Recovery” at AIChE 2019 Sustainability and Sustainable Biorefineries Poster Session.

-Published on: 11/19/2019
Congratulations Ana!
Dr. Ana Rita Colaco Morais (center front row, blue shirt) completed her post-doctoral appointment in the Shiflett laboratory and has accepted a position at the National Renewable Energy Laboratory (NREL) in Golden, Colorado. Ana will be missed by her colleagues and students, and we wish her success in her new position.

-Published on: 9/27/2019
May 2019 Commencement

Foundation Distinguished Professor of Chemical & Petroleum Engineering Mark Shiflett, right, and Tugba Turnaoglu, doctoral graduate advised by Dr. Shiflett, prepare to walk down the Hill at KU’s May 2019 Commencement. KU School of Engineering set a record for doctorates awarded in the past academic year.
Link to article.
-Published on: 9/7/2019
Congratulations Nick!
 A paper by Nick Redding, graduate student in the Shiflett Research Group, recently got accepted in I&EC Research. His paper is entitled “Mitigation of Iron and Aluminum Powder Deflagrations via Active Explosion Suppression in 1 m3 Sphere Vessel“.
A paper by Nick Redding, graduate student in the Shiflett Research Group, recently got accepted in I&EC Research. His paper is entitled “Mitigation of Iron and Aluminum Powder Deflagrations via Active Explosion Suppression in 1 m3 Sphere Vessel“.
-Published on: 8/31/2019
Congratulations Ankit!

Ankit Verma, PhD candidate in the Shiflett Research Group, published his 1st first-authored paper, entitled “Metal Recovery Using Oxalate Chemistry: A Technical Review” in I&EC Research, July 31,2019.
-Published on: 8/21/2019
Prof. Shiflett celebrates his 53rd birthday with his research team! Happy Birthday, Dr. Shiflett!

-Published on: 7/24/2019
Allgeier-Shiflett groups enjoy annual picnic together

-Published on: 7/14/2019
¡Fútbol!
The Shiflett group shared another great time together supporting the local soccer team Sporting KC against Los Angeles FC.

-Published on: 7/14/2019
Congratulations Alejandra!

Recent research published by Alejandra Rocha and Dr. Shiflett has been featured in Advances in Engineering. Their publication was based on research regarding in situ atmospheric water vapor absorption and desorption in three imidazolium-based ionic liquids, over a given range of temperatures and relative humidity conditions.
-Published on: 5/9/2019
Congratulations Dr. David Minnick!

Congratulations to Dr. David Minnick who accepted a new position at DuPont! Dave will be a Research Investigator and located at the Washington Works site in Parkersburg, West Virginia. Dave previous worked for Battelle and was a post-doctoral investigator in the Shiflett group from 2016-2018. Good luck Dave in your new career at DuPont!
-Published on: 5/9/2019
Congratulations Recently Published Researchers!
Congratulations to Dr. Minnick, Dr. Kore, and Dr. Morais for the recent publication of their research! Dr. Kore and Dr. Morais are both post-doctoral researchers with Shiflett Research Group, and Dr. Minnick previously attended KU for his graduate studies and worked in Shiflett Research Group as a post-doctoral researcher. He now works as a principal research scientist with Battelle. Read more about their research at the links below.
Minnick, D.L., Kore, R.R., Lyon, C.J., Subramanian, B., Shiflett, M.B., Scurto, A.M., Understanding Sulfur Content in Alkylate from Sulfuric Acid-Catalyzed C3/C4 Alkylations, Energy Fuels, 2019, doi.org/10.1021/acs.energyfuels.8b04364
Morais, A. C. R., Alaras, L.M., Baek, D.L., Fox, R.V., Shiflett, M.B., Scurto, A.m., Viscosity of 1-Alkyl-1-methylpyrrolidinium Bis(trifluoromethylsulfonyl)imide Ionic Liquids Saturated with Compressed CO2, J. Chem. Eng. Data, 2019, doi.org/10.1021/acs.jced.8b01237
-Published on: 4/29/2019
Congratulations Ankit Verma & Nick Redding!
Ankit Verma and Nick Redding, both graduate students in Shiflett Research Group, were recently selected for the 2019 Outstanding Graduate Student Academic Achievement Award. They will receive their awards at the annual engineering awards banquet on Saturday, May 4. Both Ankit and Nick have a 4.0 GPA in their graduate studies at KU.
-Published on: 4/24/2019
Shiflett Research Group Publishes Research
Congratulations to Tugba Turnaoglu and Ana Morais for their recent publication in the Journal of Chemical and Engineering Data. This journal article features new research and data regarding high-pressure vapor−liquid equilibrium for the binary systems of carbon dioxide (CO2) and a series of 1-alkyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide ionic liquids. Experiments were conducted using gravimetric (IGA and XEMIS microbalances) and volumetric (high pressure view cell) methods. Read more about their research in the Journal of Chemical and Engineering Data here.
-Published on: 4/24/2019
Congratulations to our recently published researchers!
Congratulations to Tugba Turnaoglu, Nick Reding, Alejandra Rocha, and Raj Kore for their recent publications! In just a short 3 months, Shiflett Research Group has featured their research in five publications in various journals. Read more about their work in their respective research fields at the links below.
Broderick, M., Rocha, M.A., Khalifa, Y., Shiflett, M.B., Newberg, J.T., Mass Transfer Thermodynamics through a Gas-Liquid Interface, Journal of Physical Chemistry, 2019, doi.org/10.1021/acs.jpcd.9b00958
Reding, N.S., Shiflett, M.B., Characterization of Thermal Stability and Heat Absorption for Suppressant Agent/Combustible Dust Mixtures via Thermogravimetric Analysis/Differential Scanning Calorimetry, Industrial & Engineering Chemistry Research, 2019, doi.org/10.1021/acs.iecr.8b06143
Kore, R., Day, V., Shiflett, M.B., Structural Identification for the Reaction of Chlorosulfonic Acid with Tertiary N-Donor Ligand — Ionic Liquid or Zwitterionic Compound?, ACS Sustainable Chemistry & Engineering, 2019, doi.org/10.1021/acssuschemeng.8b06573
Turnaoglu, T., Shiflett, M.B., The First Thermodynamic and Kinetic Analysis of Ammonia in Imidazolium Based Ionic Liquids using a Gravimetric Microbalance, Industrial & Engineering Chemistry Research, 2019, doi.org/10.1021/acs.iecr.9b00274
Rocha, M.A., Shiflett, M.B., Water Sorption and Diffusivity in [C2C1im][BF4], [C4C1im][OAc], and [C4C1im][Cl], Industrial & Engineering Chemistry Research, 2019, doi.org/10.1021/acs.iecr.8b05689
Congratulations to our researchers for recognition of their hard work!
-Published on: 3/30/19
Shiflett Research Group featured in KU News
A $1 million gift from University of Kansas alumni Richard and Elizabeth Hoover of Northport, Michigan, will establish a chemical engineering research lab at KU’s School of Engineering.
Mark Shiflett, Foundation Distinguished Professor in the Department of Chemical & Petroleum Engineering at KU, said being able to build laboratories such as the one supported by the Hoovers resonates with businesses that visit KU.
“Multibillion-dollar companies see these facilities, and they are really impressed. Companies such as DuPont and Chemours could go anywhere in the world to do research, and they come right here, to Kansas, working with us, and that offers tremendous opportunities to students,” Shiflett said. “And when projects we’ve worked on together are completed, these companies hire our students.”
This story has been featured in both KU News and the Lawrence Journal World. Read the full story by Michelle Tevis in the KU News here.
-Published on: 1/27/19
Dr. Shiflett featured as University of Delaware’s
Alumni spotlight
Congratulations to Dr. Shiflett for being featured as the alumni spotlight for the College of Engineering at the University of Delaware. Dr. Shiflett began his work with UD in 2011 as an adjunct professor in chemical engineering before coming to KU in 2016. See the full story here.
-Published on: 12/25/18
Congratulations to Dr. Shiflett!
Dr. Shiflett was recently elected as a Fellow of the National Academy of Inventors! Join us in congratulating him for such a great honor. Read the full story here.
-Published on: 12/17/18
Congratulations to Alejandra!
We would like to extend our warmest congratulations to Alejandra Rocha for successfully defending her Master’s thesis. Her presentation on December 10th was entitled “Phase Equilibria of Imidazolium-Based Ionic Liquids and Water.” We are so proud of all her hard work throughout her time working with the Shiflett Research Group!
-Published on: 12/12/18
Jayhawks at AIChE 2018
The KU reception was a hit! Thanks to all of you who could attend, can’t wait to see you again next year! Be sure to find more pictures of us in the Gallery page of our website!
-Published on: 10/30/18
See you in Pittsburgh!
The Shiflett team is excited to be attending the 2018 AIChE conference next week (see our talks below)! KU will also be hosting a reception Sunday Oct 28th at the Westin Convention Center – Westmoreland Central Room from 7:30 pm to 9:30 pm. Don’t forget to stop by!
Monday, October 29
1- Michael Shao, Mark B. Shiflett and Alejandra Rocha
Abstract Title: 106g Aspen Plus® Videos for Chemical Engineering Undergraduates
Time: 09:48-10:06 AM
Location: David L. Lawrence Convention Center-408
2-Nicholas Reding and Mark B. Shiflett
Abstract Title:189bl Characterization of Heat Absorption and Decomposition Products for Suppressant Agent/Combustible Dust Mixtures Via TGA/DSC/MS Analysis
Time: 03:30 PM – 05:00 PM
Location:David L. Lawrence Convention Center- Exhibit Hall B
3- David M. Griffin and Mark B. Shiflett
Abstract Title: 221e Integrating Laboratory Experiments into Chemical Engineering Core Courses
Time: 04:38 PM – 04:55 PM
Location:David L. Lawrence Convention Center- 411
Wednesday, October 31, 2018
4- Tugba Turnaoglu and Mark B. Shiflett
Abstract Title: 462g Solubility and Diffusivity of Ammonia in Aprotic and Protic Ionic Liquids
Time: 09:30 AM – 09:45 AM
Location:David L. Lawrence Convention Center- 316
Friday, November 02, 2018
5- M. Alejandra Rocha, S, Yong Zhang, Edward J. Maginn and Mark B. Shiflett
Abstract Title: 742a Liquid-Liquid Phase Separation of Ionic Liquids By Water Addition (Experiment and Simulation)
Time: 08:00 AM – 08:19 AM
Location:David L. Lawrence Convention Center- 306
-Published on: 10/26/18
Te Vejo em Breve Lisboa! (See you soon Lisbon!)
Dr. Shiflett will be giving a plenary lecture at EuCheMSIL in October at Lisbon, Portugal. If you will be attending, be sure to stop by and say hi! More info here.
-Published on: 9/17/18
¡Fútbol!
The Shiflett group shared a great time together supporting the local soccer team Sporting KC against Orlando City. And yes … of course SKC won!!

-Published on: 9/9/18
Upcoming Publications
Be on the lookout for papers from our group in press soon!
Nick Reding and Dr. Shiflett ‘s paper: Metal Dust Explosion Hazards: A Technical Review was accepted into the Industrial & Engineering Chemistry Research Journal.
Dr. Minnick, Alejandra Rocha, Tugba Turnaoglu, and Dr. Shiflett ‘s paper: Gas and Vapor Sorption Measurements using Electronic Beam Balances was accepted as a featured article in the Journal of Vacuum Science & Technology A.
-Published on: 8/11/18
Ringing Bells!
The KU-rooted pharmaceutical company Savara went public on NASDAQ May of 2017, and last month had the great opportunity to ring the market bell in NYC on July 23rd. Our own research associate, Dr. Cory Berkland (profile), was actually part of the creation of Savara, which he started out of his lab in 2007 to develop aerosolized drug therapies. Read more here!

-Published on: 8/11/18
Congrats to Ana and the Vaccine Team!
Ana C Morais was recently awarded the 2018 Baxter Young Investigator award for her work on the vaccine project. Rock Chalk! Follow this link for more info on the advancements the Shiflett group is making on the thermal stabilization of vaccines.
-Published on: 8/1/18
Summer Research!
For many of our undergrads summer and research go hand in hand, and several of them had the opportunity to present their work at the KU 2018 Summer Poster session. See their pictures below 🙂 !
-Published on: 7/27/18
Fire Training
This summer, Simon Velasquez, one of Dr. Allgeier grad students, set up an instructive hands-on activity for our high school intern: fire extinguisher safety! Thanks Simon!
-Published on: 7/16/18
It’s Summer!
The Shiflett Research group and Allgeier Research group enjoyed a fun summer cookout at Clinton Lake and showed off their impressive sand volleyball skills! More pictures in our Gallery page!
-Published on: 7/15/18
Up with Teaching, Down with CO2
Dr. Mark Shiflett is working to elevate the research and educational opportunities of KU’s new Central Utility Plant (CUP), which produces hot and chilled water for KU’s Central District, while reducing KU’s carbon footprint and creating stronger ties with industry. Read more here!
-Published on: 7/03/18
“Boulder” Jayhawks!
Tugba, Alejandra, and Dr. Shiflett gave great talks and poster presentations at the 20th Symposium of Thermophysical Properties at the University of Colorado Boulder! See the list below!
Tugba Turnaoglu
Oral Presentation: A Kinetic and Thermodynamic Analysis of Ammonia in Ionic Liquids
Poster: Liquid-Liquid Equilibria of 1,5-Pentanediol in Room Temperature Ionic Liquids
Alejandra Rocha
Oral Presentation: Vapor-Liquid-Liquid Equilibria of Ternary Imidazolium-based Ionic Liquids and Water Systems
Poster: Water Sorption in Ionic Liquids Characterized using a Dynamic Vapor Sorption Analyzer (IGAsorp) and Ambient Pressure X-ray Photoelectron Spectroscopy (APXPS)
Dr. Mark Shiflett
Oral Presentation: Investigating the Role of Atom Substitution on the Hydrogen Bonding Interactions and Solubility of Hydrofluorocarbons in Ionic Liquids
Bonus picture!

-Published on: 6/28/18
Welcome to Our 3 New Post Doctoral Researchers!
Dr. Ana Morais, Dr. Rajkumar Kore, and Dr. Sachin Nandanwar have joined the group beginning this summer. We give them a warm welcome and look forward to celebrating their future accomplishments with the team! Learn more about our new members here.
-Published on: 6/07/18
Congrats to the Happy Couples!
May has been full of big dates with several of our undergrads graduating, Bill and Dave saying bye to the team, welcoming new post-docs, and people getting married!
Bill and Tara celebrated their marriage on Saturday May 12th in Lawrence, KS and we wish them the best on this wonderful journey together!
Alejandra and Jason also tied the knot in Lawrence on Friday May 18th; we wish them a lifetime of love and happiness!
-Published on: 6/07/18
Bidding Farewell to Dave and Bill
Dr. Dave Minnick and Dr. Bill Gilbert will be departing the lab group and starting their new jobs in industry within the coming weeks. We will miss them greatly, but we wish them the best on their new careers!


-Published on: 5/10/18
Thank you!
Our sponsor network continues to expand, and we are honored to have their support! Our list can be found on our Sponsors tab. Feel free to check them out!
-Published on: 4/7/18
Congratulations to Tugba on her Award!
Tugba Turnaoglu, along other KU CPE researchers, attended the 2018 Capitol Graduate Research Summit which was held at the State Capitol, Topeka, on the 27th of March, 2018. She received the BioKansas Presentation Award at the Summit. Yay, Tugba!! For more info please see here.
-Published on: 3/30/18
Rock Chalk, Tugba!
Great news keep coming for our team! Tugba Turnaoglu passed her comprehensive exam today! Her topic was “Protic Ionic Liquid-Zeolite Composites for Ammonia Separation in the Haber-Bosch Process.” Congratulations, Tugba!!
-Published on: 3/26/18
Congratulations to Katie!
Katie Bauguess has accepted a full time position with ExxonMobil. She will be working as a contact engineering in their Baton Rouge, LA site beginning August, 2018.
-Published on: 2/28/18
Congrats Dave!
Dr. David Minnick recently accepted a principal research scientist position with Battelle. Dave will begin working at the Battelle Eastern Science and Technology Center located in Aberdeen, MD starting in June, 2018.
-Published on: 2/8/18
Congrats Bill!
Congratulations to Dr. Bill Gilbert who accepted an offer of employment from the Chemours Chemical Company. Bill will be working at the Chemours TiO2 facility in New Johnsonville, Tennessee. He will start in June 2018.
-Published on: 12/2/17
Wine Science
Shiflett lab is on the news again! Check out the KU article featuring undergraduate Maddie Lyda, Dr. Bill Gilbert, and Dr. Mark Shiflett on researching sulfite removal from wine. Our group hopes to develop and market an inexpensive device which can remove 99% of sulfites from wine.

-Published on: 11/29/17
Part of a Great Network
Dr. Mark Shiflett recently completed a news story on ionic liquid solubilities with AZO Network,a system focused on “Telling science, technology and medical stories to people who can make a difference.” In the piece, Dr. Shiflett discusses the different applications of ionic liquids and describes the importance in measuring gas solubilities.
Check it out here!
-Published on: 11/25/17
AIChE Annual Conference Successes!
Our group is very grateful to have presented at the 2017 AIChE Annual Conference. We had over 8 talks and posters representing the Shiflett group. Congrats to Brooks Danahy for earning an award on his poster at the undergraduate session!
And, the AIChE hospitality suite was a success! We look forward to seeing you again next year!


-Published on: 11/12/17
KU Chemical & Petroleum Engineering Hospitality Suite
Will you be at the annual AIChE conference in Minneapolis this year? Join us at our hospitality suite and learn about our novel research and cutting-edge facilities!
When: 7:30PM-9:30PM on Sunday, October 29th
Where: Duluth Room, Hilton Minneapolis, 3rd floor near escalators
We look forward to meeting you!

-Published on: 10/05/17
On the Cover!
The book Ionic liquids: Current State and Future Directions edited by Dr. Scurto and Dr. Shiflett, was published recently. And of course, the book (bottom left image) displayed their KU pride with crimson and blue on the cover! In addition, Dr. Shiflett wrote a perspective article in the November issue of the AIChE Journal, and it is featured on the cover (bottom right image).
-Published on: 9/26/17
Presence Abroad!
Dr. Mark Shiflett was invited to present a keynote lecture at the European Conference on Thermophysical Properties in Graz, Austria. He recently traveled to Graz, and presented his a talk “Ionic Liquids – Phase Behavior to Applications” on the morning of Tuesday September 5th.
Later that week, Dr. Shiflett also had the opportunity to visit the Hiden Isochema Ltd. headquarters in Warrington, UK to celebrate 15 years of successful collaboration. Dr. Mark Roper, Director of Sales and Marketing at Hiden, met with Dr. Shiflett and provided a tour of Hiden’s advanced instrumentation.
-Published on: 09/16/17
Future Conferences
We are excited to have our graduate students and postdoctoral researchers presenting at two upcoming conferences. Tugba, Alejandra, Bill, and Dave will be presenting posters at the upcoming 2017 ACS Midwest Regional Meeting – Catalysis Symposium at KU on October 19th, and oral presentations at the 2017 AIChE Annual Meeting in Minneapolis on October 29th – November 2nd. Be sure to stop by if you are attending the conferences!
Details below:
2017 ACS Midwest Regional Meeting
Tugba Turnaoglu:
Vapor-Liquid Equilibria:Ammonia and 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide
Friday October 20th, 12:30PM-2:30PM
Alejandra Rocha:
Clathrate Hydrate Formation Using Fluorocarbons
Friday October 20th, 12:30PM-2:30PM
Bill Gilbert:
Safety Considerations when Designing a New Chemical Engineering Research Laboratory
Friday October 20th, 8:00AM-10:00AM
Dave Minnick:
Solubility of vinyl fluoride in aqueous lithium bis(trifluoromethylsulfonyl)imide solutions
Friday October 20th, 12:30PM-2:30PM
2017 AIChE Annual Meeting (at the Minneapolis Convention Center)
Tugba Turnaoglu:
-Ammonia Absorption in Ionic Liquids
Wednesday November 1st, 9:42 AM – 9:59 AM, Room 103B
Alejandra Rocha:
-Clathrate Hydrate Formation Using Fluorocarbons
Tuesday October 31st, 8:51 AM – 9:08 AM, Room L100I
-Water Sorption in Ionic Liquids Characterized Using a Dynamic Vapor Sorption Analyzer (IGASorp) and High Pressure X-Ray Photoelectron Spectroscopy (APXPS)
Thursday, November 2nd, 4:00 PM – 04:15 PM, Room M100B
Bill Gilbert:
-Safety Considerations When Designing a New Chemical Engineering Research Laboratory
Monday October 30th, 1:42 PM – 2:00 PM, Room 205C
-Desorption of Gases from Ionic Liquids Using an Applied Electric Field
Tuesday October 31st, 12:30 PM – 12:52 PM, Room 101C
Dave Minnick:
-Measurement and Modeling of Vinyl Fluoride Solubility in Aqueous Lithium Bis(trifluoromethylsulfonyl)Imide Solutions
Monday October 30th, 12:50 PM – 01:10 PM, Room M100C
-Phase Behavior of Compressed Gases in Ionic Liquids at the Liquid-Solid Transition Point
Tuesday October 31st, 04:12 PM – 04:31 PM, Room L100J
Shiflett Lab Group Hosts Open House
Shiflett lab group hosted an open lab afternoon on April 28. The event featured undergraduate research presentations on their different projects from this semester. Dr. Shiflett and our post doctorate researchers also gave visitors tours of the lab space.
New Kinetics Experiment for Undergraduate Chemical Engineering Laboratory
Dr. David Griffin, Dr. Bill Gilbert and Prof. Mark B. Shiflett

A new kinetics experiment was completed and added to the undergraduate chemical engineering teaching laboratory for the Chemical Engineering Laboratory I, C&PE 616. Students can measure the kinetic orders and activation energy for the iodination of acetone reaction (CH3COCH3 + I2 → CH3COCH2I + HI) using HCl as a catalyst.
The reaction is a color change reaction so it starts as a colored solution when iodine is added (I2 is red/brown in color) and goes clear as the product iodoacetone is formed (iodoacetone is clear). The experimental setup consists of a 1 liter stirred tank reactor with a cooling/heating jacket controlled with a Peltier temperature bath. A pump circulates a sample of the reactor fluid through a flow cell in a spectrometer for instantaneous analysis of the iodine concentration as a function of time. The temperature and absorbance are recorded using a LabView® data acquisition program on a laptop computer. The waste can be drained to waste containers beneath the reactor and the system is mounted on a moveable rack which allows the experiment to be portable. The setup is identical on both sides of the rack so that students can work in groups of two to run both reactors simultaneously. The spectrometer measures the absorbance of iodine at a wavelength of 510 nm. Students use both a linear regression (Initial Rates Method) and a non-linear regression to analyze the kinetics data to determine the orders of reaction and the activation energy. In addition to the experiments, students model the reaction using Aspen Plus using both batch (RBATCH) and continuous (RCSTR) reactor models. The fact that the experiment is portable allows the equipment to be wheeled into the classroom for demonstration during lectures. Students taking Chemical Kinetics C&PE 524 will learn how the experiment works, the methods for data analysis and be assigned a homework set based on actually experimental data being measured by the Senior class. This will connect the lab course with the kinetics course and familiarize the Juniors in C&PE 524 with the kinetics experiment they will do the following semester in the undergraduate laboratory course C&PE 616. In addition, future experiments can be conducted with other reactants such as bromine and the effect of different catalysts can be studied.
A new thermodynamics experiment is planned for construction during the summer of 2017 and ready for use in C&PE 616 by the Fall semester. The intent is by the Fall of 2018, that three new experiments focused on kinetics (complete), thermodynamics and fluid mechanics will be ready which connect the theory taught in the core courses with the laboratory course. The experiments will be portable and allow instructors to describe the experiments in the classroom and build homework sets based on data measured by students to familiarize Juniors with the Senior laboratory equipment and analysis methods.
-Published on: 03/03/17

















































You must be logged in to post a comment.