Prof. Shiflett teaches the Undergraduate Chemical Engineering Senior Laboratory courses, C&PE 616 (Laboratory I) and C&PE 626 (Laboratory II) in the Fall and Spring semesters.

Prof. Shiflett redesigned the courses with a new format and experiments. The new format incorporates prelab tours, prelab meetings, experiments, data analysis, reports, oral presentations with opportunities for students to make videos for instructional guidance to future students. An example of the new format for the Monday lab groups is shown below.

The first experiment that Prof. Shiflett designed was a new Kinetics Experiment in 2017.
New Kinetics Experiment for Undergraduate Chemical Engineering Laboratory
Dr. David Griffin, Dr. Bill Gilbert and Prof. Mark B. Shiflett.
A new kinetics experiment shown in Figure 1 was completed and added to the undergraduate chemical engineering teaching laboratory for the Chemical Engineering Laboratory I, C&PE 616. Students can measure the kinetic orders and activation energy for the iodination of acetone reaction (CH3COCH3 + I2 → CH3COCH2I + HI) using HCl as a catalyst.

The reaction is a color change reaction so it starts as a colored solution when iodine is added (I2 is red/brown in color) and goes clear as the product iodoacetone is formed (iodoacetone is clear). The experimental setup consists of a 1 liter stirred tank reactor with a cooling/heating jacket controlled with a Peltier temperature bath. A pump circulates a sample of the reactor fluid through a flow cell in a spectrometer for instantaneous analysis of the iodine concentration as a function of time. The temperature and absorbance are recorded using a LabView® data acquisition program on a laptop computer. The waste can be drained to waste containers beneath the reactor and the system is mounted on a moveable rack which allows the experiment to be portable. The setup is identical on both sides of the rack so that students can work in groups of two to run both reactors simultaneously. The spectrometer measures the absorbance of iodine at a wavelength of 510 nm. Students use both a linear regression (Initial Rates Method) and a non-linear regression to analyze the kinetics data to determine the orders of reaction and the activation energy. In addition to the experiments, students model the reaction using Aspen Plus using both batch (RBATCH) and continuous (RCSTR) reactor models. The fact that the experiment is portable allows the equipment to be wheeled into the classroom for demonstration during lectures. Students taking Chemical Kinetics C&PE 524 will learn how the experiment works, the methods for data analysis and be assigned a homework set based on actually experimental data being measured by the Senior class. This will connect the lab course with the kinetics course and familiarize the Juniors in C&PE 524 with the kinetics experiment they will do the following semester in the undergraduate laboratory course C&PE 616. In addition, future experiments can be conducted with other reactants such as bromine and the effect of different catalysts can be studied.
The second experiment Prof. Shiflett designed was a new Thermodynamics Experiment in 2018.
New Thermodynamics Experiment for Undergraduate Chemical Engineering Lab
Dr. David Griffin, Mr. George Whitmyre, Prof. Mark Shiflett

A new thermodynamics experiment was designed and constructed for the Fall, 2017 undergraduate Chemical Engineering Laboratory, C&PE 616. Designing a VLE-based separation process requires information about the compositions of phases (vapor and liquid) as a function of temperature, pressure, and overall composition. The objective of this experiment is to obtain data so that the students can predict the isobaric vapor-liquid equilibrium for a particular ternary mixture by measuring the VLE in the constituent binary mixtures. The students learn to operate four modified Swietoslawski ebulliometers which operate at ambient pressure for measuring the boiling temperature for pure component and mixtures to calculate activity coefficients near the limits of infinite dilution.
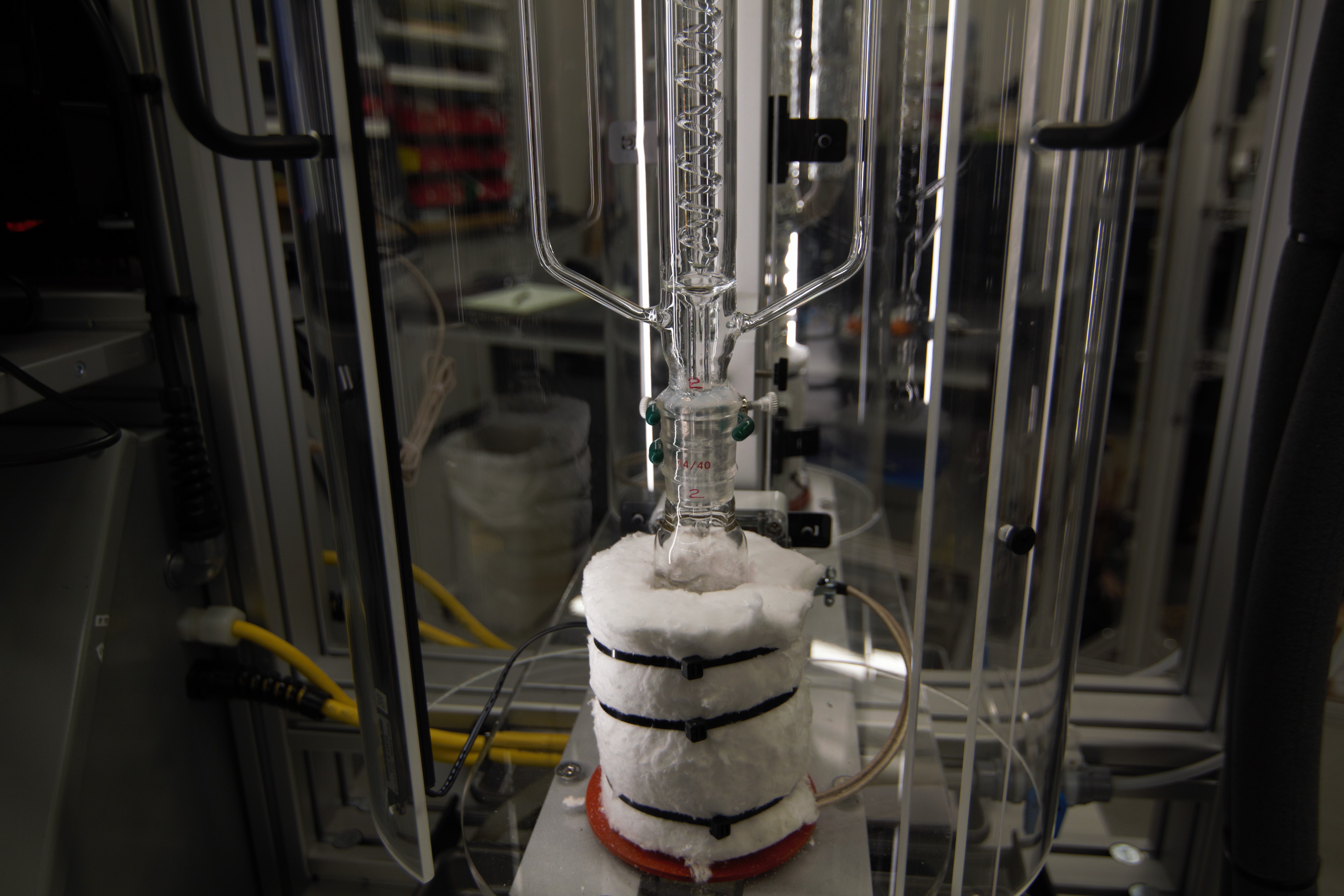
The infinite dilution activity coefficients, obtained by data regression, will be used in common activity coefficient models (NRTL, Wilson, Margules) to predict the activity coefficients over the whole concentration range, and then for the construction of binary phase diagrams. The predictive capabilities of the Regular Solution activity coefficient models will be tested for the binary systems and compared with known literature values. Based on these results, the students choose the best model for the prediction of the ternary VLE phase behavior. Results from the isobaric experiment allow construction of useful x-y and T-x-y phase diagrams and solving a design problem which requires an isothermal or adiabatic flash calculation. Students are also introduced to ASPEN Plus (version 10) process simulation software to perform flash calculations. For a discussion of VLE phase diagrams, see S. I. Sandler, Chemical, Biochemical and Engineering Thermodynamics, 5th Ed., J. Wiley and Sons, 2017.
The third experiment that Prof. Shiflett designed was a new Fluid Mechanics Experiment in 2019.
New Fluid Mechanics Experiment for Undergraduate Chemical Engineering Lab
Dr. David Griffin, Mr. George Whitmyre, and Prof. Mark Shiflett

Fluid Mechanics experiment: Electric centrifugal pumps (A) move water from the tank (B) through the five pipe attachments with flow isolation controlled by ball valves (C). A differential pressure transducer (D) measures the pressure drop between two taps located on the pipes. A laptop computer (E) displays and records data from the pressure transducer and flowrate sensors.
One side of the fluid mechanics experiment is setup for measuring friction loss through pipes at low flow rate conditions and the other side is setup for measuring friction loss through pipes at high flow rate conditions. The setup consists of centrifugal pumps, variable frequency drive (VFD) motor, pipes of various materials and diameters, pressure transducers, and flowmeters. A computer with LabView software is connected to the pressure transducers and flowmeters to record the pressure and flowrate at any given time interval for a selected pipe material. Additional equipment parts consist of such as control valves, quick connect couplers, water storage tank, and multiple storage racks for use as required. It is essential to maintain a completely horizontal pipe scheme in these experiments. The two symmetrical setups are used for obtaining data in two different flow regimes (laminar and turbulent). The equipment is used to experimentally observe pressure drop as a function of fluid velocity over a wide range to cover laminar to turbulent regimes. These data are obtained for different pipe materials with different diameters.
The fourth experiment that Prof. Shiflett designed was a new Controls Experiment in 2021.
New Controls Experiment for Undergraduate Chemical Engineering Lab
Dr. Felipe Anaya, Dr. David Griffin, Mr. George Whitmyre, and Prof. Mark Shiflett

The objective of the new Control Experiment is to tune a PID controller regulating liquid level in a water tank with either a pneumatic diaphragm control valve or variable speed pump. Controller parameters (KC, τI, τD) will be determined experimentally from standard industrial heuristics (IMC, IAE, ITAE). In addition, students use a first principles approach to estimate the system transfer function and compare to experimental results. Students operate the apparatus – consisting of two tanks with a single inlet and a single outlet, and one mixing tank fed from both tanks with a single outlet – to test the effectiveness of initial controller parameters and to control the system following a disturbance. Further experimentation can be carried out to systematically determine the effect of each parameter on the operation of the control system. Simulation software (e.g., Simulink) is employed to model the dynamic system and compare the simulation to experimental results.
The fifth experiment that Prof. Shiflett designed was a new pilot-scale Methanol/Water Distillation Experiment in 2022.
New Distillation Experiment for Undergraduate Chemical Engineering Lab
Dr. Felipe Anaya, Dr. Michael Lundin, Mr. Ethan Finberg, Mr. Ed Atchison, Mr. Dan Truitt, Mr. George Whitmyre, and Prof. Mark Shiflett


The separation of mixtures into pure components is of central importance in the chemical industry. The objective of the new Distillation Experiment is to teach students how to operate a pilot-scale, distillation column to separate a mixture of methanol and water into pure components. Students utilize experimental vapor-liquid equilibria (Txy) data to design a process for separation of methanol and water; perform experiments to demonstrate proficiency in the operation of a pilot-scale distillation column; model and validate experimental results using ASPEN Plus simulation, carry out material and energy balances; and solve a design problem using ASPEN Plus to analyze a “real world” separation problem.
The sixth experiment that Prof. Shiflett designed was a new pilot-scale Extractive Distillation Experiment in 2022.
New Extractive Distillation Experiment for Chemical Engineering Research
Dr. Michael Lundin, Mr. Ethan Finberg, Mr. Ed Atchison, Mr. Dan Truitt, Mr. George Whitmyre, and Prof. Mark Shiflett


Some mixtures that form azeotropes cannot be separated using conventional distillation techniques; therefore, a new extractive distillation column was designed to study the separation of refrigerant mixtures using ionic liquids as the entrainer. This is the world’s first extractive distillation column for separation of azeotropic refrigerant mixtures and is currently being used for research to develop the process for commercialization. The extractive distillation column is currently developing entrainers for separating R-410A that is a mixture of 50 wt% difluoromethane (HFC-32) and 50 wt% pentafluoroethane (HFC-125) into pure refrigerants (>99.5 wt%) so that the HFC-32 can be recycled into new products and the HFC-125 can be repurposed. Ultimately, the extractive distillation process will also be used for undergraduate education in chemical engineering.
New Control Room for Undergraduate Chemical Engineering Lab
Dr. Felipe Anaya, Dr. Michael Lundin, Mr. Ed Atchison, Mr. Dan Truitt, Mr. George Whitmyre, and Prof. Mark Shiflett

A state-of-the-art control room has been designed for students to operate both the distillation column and extractive distillation column. The facility provides a “real-life” experience for students to see what it feels like to operate pilot-scale equipment from a control room setting. The control room was designed so that students could see into the high-bay area where the pilot-scale processes are setup. Students can operate the equipment using LabView software on the computer, from the control cabinet which uses Allen Bradley hardware and software, or from a tablet that can be carried with them into the high-bay area. The control room is setup for operating three experiments simultaneously and a third experiment is being designed for conducting sorption experiments in the future.
ASPEN Plus® Tutorials
Taught ASPEN Plus® modeling using video presentations at the University of Kansas and University of Delaware. Video library includes: Hydrogenation of Benzene to Cyclohexane (KU).
C&PE 661 Undergraduate Honors Research
Advise undergraduates in chemical engineering thermodynamic and reaction engineering experiments with my graduate and post-doctoral researchers
C&PE 651 Undergraduate Research
Advise undergraduates in chemical engineering thermodynamic and reaction engineering experiments with my graduate and post-doctoral researchers
University of Kansas Central Utility Plant Powers Unique Learning Opportunities
Adjacent to the University of Kansas Integrated Science Building (ISB) is KU’s new Central Utility Plant (CUP), which produces hot and chilled water for the KU’s Central District, which includes the ISB, a new Burge Union and two residence halls. The $15 million CUP was designed to provide utilities and educational opportunities for student research and includes a classroom — complete with windows looking into the machine room — for faculty and students to work closely with industry partners and provide opportunities for outreach with other universities and educators.
Foundation Distinguished Professor of Chemical & Petroleum Engineering Mark Shiflett is working to elevate the research and educational opportunities of this space, while reducing KU’s carbon footprint and creating stronger ties with industry.
“Our proposal is to provide a location for companies to test bench-scale to pilot-scale CO2 capture and conversion technologies using ‘real’ flue gas in a modern power plant designed for research and education.”
“The facility will provide the opportunity for faculty, graduate, and undergraduate students at KU to study alongside industry scientists exploring how to reduce the impact of CO2 on our environment. The University of Kansas would be the first university to install CO2 systems in their own power plant for research, education and reducing carbon footprint.”
Shiflett’s vision includes retrofitting one of the chillers with a new low-global warming refrigerant so students can study the cooling capacity, energy efficiency and emission differences while working alongside industry scientists from the refrigerant and chiller manufacturer.
The enhanced learning environment Shiflett proposes also includes benefits to students beyond those studying engineering or other hard sciences.
“Humanities students will also be exposed to authentic research to understand how public policies intersect with technological innovation and the challenges to implementation.”
Prof. Shiflett is working with industry partners to retrofit the chiller this Fall and is in discussions with Oil & Gas companies about piloting CO2 capture and conversion technologies.
Foundation Distinguished Professor (2016-Present)
University of Kansas
Department of Chemical and Petroleum Engineering
ME Capstone Project: KU Central Utility Plant Chiller Retrofit
The new central utility plant (CUP) at the University of Kansas has two 900 ton York chillers that will provide chilled water for cooling the new Integrated Science Building (ISB), Kansas Student Union and Central District. The chillers will initially operate using refrigerant R-134a (1,1,1,2-tetrafluoroethane). A mechanical engineering Capstone project led by Cade Albert, Chad Sevart, Michel Alhathal, Josh Seybert and Yong Hahn (shown standing in front of the chillers) and supervised by Prof. Mark Shiflett (Chemical Engineering) and Prof. Tom DeAgostino (Mechanical Engineering) are measuring the cooling capacity and energy efficiency of R-134a to determine the baseline chiller performance. Plans are to retrofit one of the chillers to a new lower global warming refrigerant in the Fall of 2018 to measure the differences in cooling capacity and energy efficiency relative to the R-134a baseline measurements.

Adjunct Professor (2011-2016)
University of Delaware
Department of Chemical and Biomolecular Engineering
CHEG 345 Chemical Engineering Laboratory I
Junior Lab, kinetics and vapor-liquid equilibria theory and experimentation, and safety
CHEG 445 Chemical Engineering Laboratory II
Senior Lab, distillation theory, operation of pilot-scale column, and process hazards analysis
CHEG 473/474 Junior/Senior undergraduate research
Advised 39 students during the past six years to: design new kinetics experiment for Junior lab, reactivate heat-exchanger experiment for Junior lab, upgrade pilot-scale distillation column from batch to continuous operation for Senior lab, create ASPEN tutorial videos for introductory course in ChE, measure friction and wear properties for bioderived and synthetic lubricants, and publish manuscripts.
UNIV 401/402 Undergraduate Thesis
Advised undergraduate thesis student, obtained departmental research funding, Project: Friction and Wear Characteristics for Bioderived Lubricants – Advantages versus Synthetic Lubricants.
CHEG 112 Material and Energy Balances
ASPEN modeling



You must be logged in to post a comment.